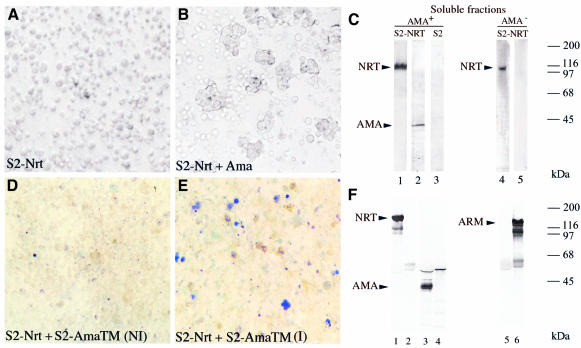
Fig. 2. AMA is required for aggregation of NRT transfectants. NRT transfectants expressing high levels of NRT protein did not aggregate spontaneously (A); however, if they were incubated with the 100 000 g supernatant, they formed homotypic aggregates (B). Protein analysis of the corresponding aggregates or cells is shown in (C). Soluble fractions used as a source of ligands were obtained from either wild-type (lanes 1–3) or ama-deficient (lanes 4 and 5) embryos. NRT transfectants were incubated with the wild-type soluble fraction to allow aggregation. Aggregates were analyzed by SDS–PAGE/western blotting with anti-NRT antibodies (lanes 1–4) or anti-AMA antibodies (lanes 2–4). NRT transfectants remained as single cells when they were incubated with AMA-deficient soluble fraction. Untransfected S2 cells (lane 3) did not bind AMA protein; however, staining for a longer time shows a very weak signal due to the endogenous AMA expression. S2 AMA-TM transfectants were grown to confluence on slide flasks and exposed to bivalent cations to induce AMA-TM expression. Incubation at 25°C for 1.5 h allowed full AMA-TM expression, then the cell monolayer was overlaid with a suspension of methylene blue-stained NRT transfectants (Barthalay et al., 1990). After a 15 min incubation and several gentle washes, the slides were examined by microscopy. (E) A field with blue NRT-expressing cells bound on unstained AMA-TM transfectants (I: induced). (D) The same experiment where expression of AMA-TM was not induced (NI). (F) A co-immunoprecipitation assay. Anti-NRT antibodies (lanes 1, 3 and 5) or anti-ARM antibodies (lanes 2, 4 and 6) were added to embryo lysates that were incubated with protein A–Sepharose. Immunoprecipitates were analyzed on SDS–PAGE, then blotted onto nitrocellulose and probed with anti-NRT (lanes 1 and 2), anti-AMA (lanes 3 and 4) and anti-ARM antibodies (lanes 5 and 6).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.