Figure 10.
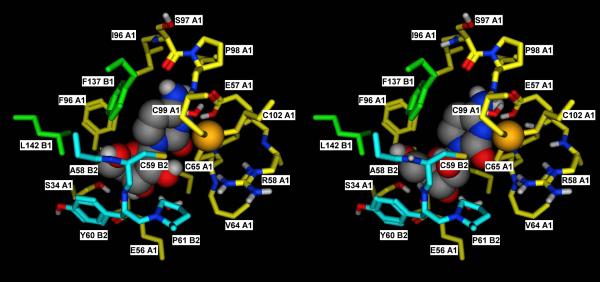
A) Detailed view of the catalytic site of subunit A1 in the crystal structure of the mouse cytidine deaminase in complex with cytidine (2FR6, (Teh et al., 2006)).
B) Corresponding docking complex obtained in silico for Ara-C. The catalytic importance of the tetrameric structure derives from the fact that residues of subunits B1 and B2 contribute to the formation of the catalytic site. The docking complex obtained for Ara-C suggests that the orientation that the 2'-OH group assumes in this compound may exert some steric hindrance with the residues of subunit B2, thus adding a destabilizing element for the quaternary structure. In the Y33 mutant, this is probably sufficient to disrupt the already weak tetramer, thus explaining its inability to catalyze the deamination of Ara-C.
Subunit A1, B1 and B2 are in yellow, green and cyan, respectively. Residues lining the binding pocket are shown in sticks representation, with the carbon atoms colored by subunit, and the heteroatoms colored by element. Cytidine and Ara-C are shown in space filling representation, with the atoms colored by element.