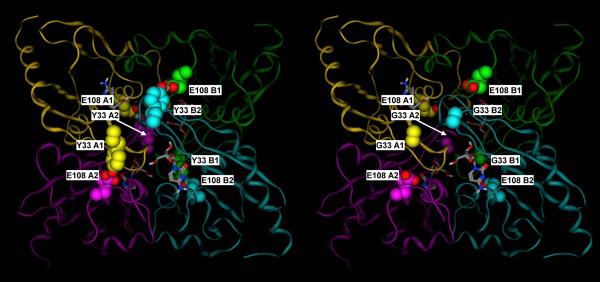
Figure 9.
A) Crystal structure of the mouse cytidine deaminase in complex with cytidine (2FR6, (Teh et al., 2006).
B) Corresponding molecular model of the Y33 mutant. Y33 of subunits A1, A2, B1 and B2 form hydrogen bonds with E108 of subunits A2, A1, B2 and B1, respectively, thus double latching each of the two monomer pairs corresponding to the catalytic site and the C-terminal broken site in the E. coli dimeric enzyme (A1–A2 and B1–B2). Subunit A1, A2, B1 and B2 are in yellow, purple, green and cyan, respectively. Y33 and E108 residues are shown in space filling representation, with the carbon atoms colored by subunit, and the heteroatoms colored by element. Cytidine is shown in sticks representation, with the atoms colored by element.