Figure 1.
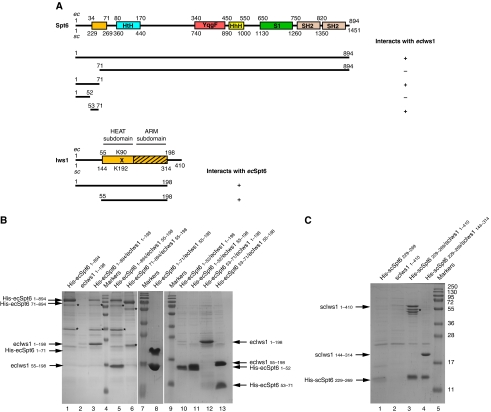
A small N-terminal region of Spt6 is sufficient to retain the full length or the conserved domain of Iws1. (A) Schematic view of the putative domain architecture of Spt6 and Iws1. The domains of Spt6 and Iws1 characterized in this study are shown as light orange boxes. This domain in Iws1 is composed of two subdomains (HEAT subdomain and ARM subdomain) and corresponds to the conserved region of Iws1 sufficient for Iws1 function in yeast (Fischbeck et al, 2002). The Iws1 invariant lysine (scK192/ecK90) involved in the Spt6-independent function of Iws1 is marked by an ‘X'. The Iws1 region homologous to TFIIS, Elongin A and Med26 N-terminal domains is hatched. The domains (HtH, YqgF, HhH and S1) that have been putatively assigned to the Spt6 core domain based on the structure of the bacterial Tex protein (Johnson et al, 2008) are shown. The two SH2 domains from the tandem SH2 domains at the C-terminus of Spt6 (Diebold et al, 2010; Sun et al, 2010) are indicated. The results of co-expression experiments shown in (B) are summarized below the proteins. E. cuniculi (ec) and S. cerevisiae (sc) numbering are shown. (B) Deciphering of E. cuniculi Iws1/Spt6 complex formation upon (co-) expression in E. coli of various constructs of both proteins and purification by affinity chromatography. All samples are analysed on SDS–PAGE. The fainter band for His-ecSpt6N53−71 in lane 12 compared with lane 13 is due to the lower amount of soluble complex obtained. Construct boundaries are indicated. Spt6 degradation products are marked with an ‘*'. (C) Characterization of the S. cerevisiae Iws1/Spt6N interaction based on the data obtained with the E. cuniculi proteins. The faint band for His-scSpt6N229−269 in lane 1 is most likely due to the poor solubility of this construct when expressed alone. Iws1 degradation products are marked with an ‘*'. Molecular weights are shown and are the same throughout the figures.