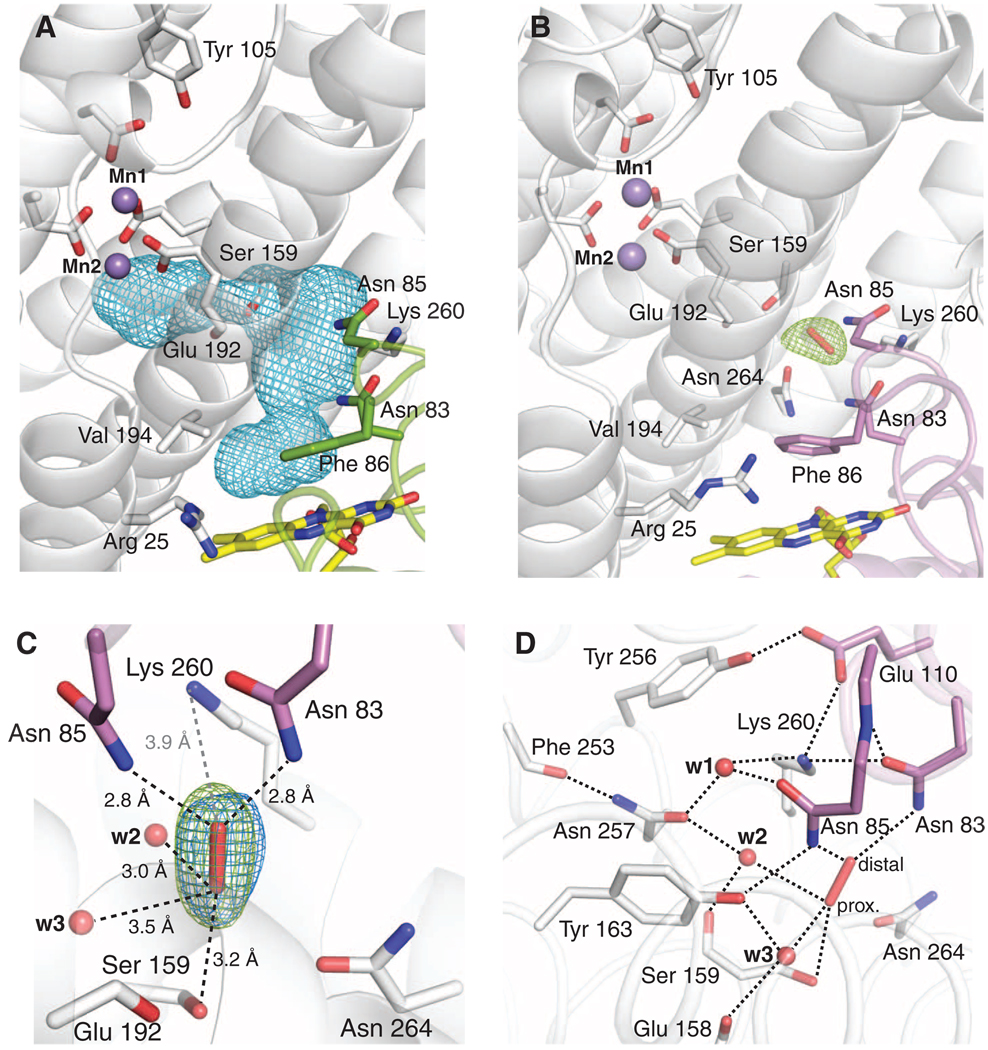
Fig. 4.
The NrdI-NrdF channel. (A) NrdI-NrdF complex formation extends the NrdF active-site channel to the FMN cofactor. The complex channel is depicted as a light blue mesh and was calculated using a 1.4 Å probe radius. Selected NrdI (green) and NrdF (white) residues lining the channel are shown as sticks. (B) Observation of a trapped species, best modeled as peroxide, in the NrdI-NrdF channel in a crystal reduced by dithionite in the presence of oxygen (NrdIhq-NrdFperox). Strong Fo − Fc electron density (green mesh, contoured at 3.3σ) is present in the channel after the first refinement cycle. The FMN cofactor (yellow), NrdI side chains lining the channel (purple), NrdF residues in the channel and at the active site (white), and the peroxide (red) are all shown as sticks. (C) A magnified view of the modeled peroxide shown in Fig. 3B and hydrogen bonding interactions with residues and solvent in the channel. The final 2Fo − Fc electron density (blue mesh, contoured at 1.8σ) is superimposed on the initial Fo − Fc electron density map from Fig. 3A. Water molecules are shown as red spheres. Dashed black lines indicate potential hydrogen bonding interactions. The gray dashed line represents the distance between the modeled peroxide and the nearest charged residue, conserved NrdF residue Lys260. The Glu192 backbone carbonyl group and the side chain of Ser159 constitute the narrowest point of the active-site channel. The oxygen atom distal to the MnII2 site interacts strongly with the side chains of NrdI residues Asn85 (2.8 Å) and conserved Asn83 (2.8 Å). (D) The extended hydrogen bonding network near the putative peroxide binding site. The side-chain orientations of Asn83 and Asn257 can be assigned unequivocally based on their interactions with Lys260 and the backbone amide nitrogen of Asn85, and the carbonyl oxygen of Phe253, respectively. The interactions of w2 with Asn257 (2.8 Å) and the backbone carbonyl of Ser159 (2.7 Å) constrain w2 to act as a hydrogen bond acceptor for the proximal oxygen atom of the modeled peroxide (2.9 Å), suggesting that this oxygen is protonated. The distal oxygen accepts two hydrogen bonds from Asn83 and Asn85. Because no other potential hydrogen bond donor or acceptor exists for this oxygen atom, its protonation state cannot be determined from this analysis.