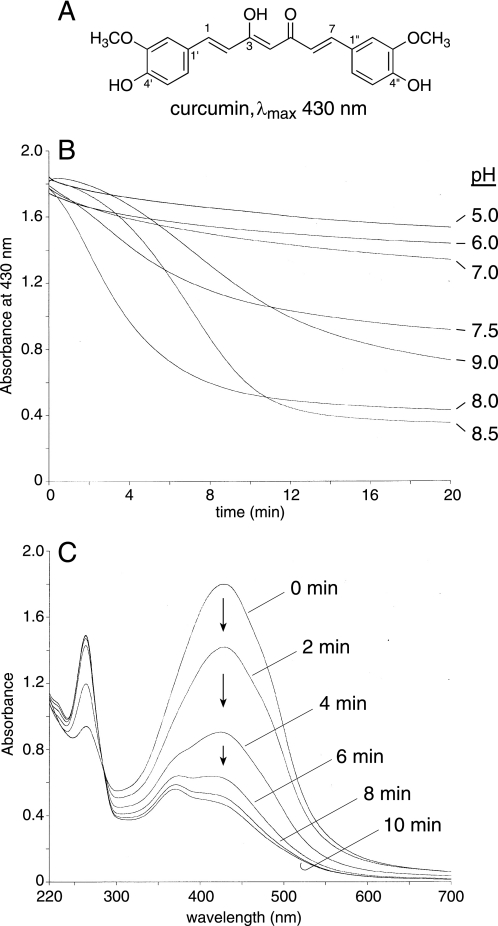
FIGURE 1.
Effect of pH on the transformation of curcumin. A, extended conjugation of the seven-carbon heptadienone chain connecting the two phenolic rings results in the yellow-orange color of curcumin. In 4′-methoxy- and 4′,4′-dimethoxycurcumin the phenolic hydroxyl group is replaced by a methoxy group. B, curcumin was added to buffer of the pH indicated and the decrease in absorbance at 430 nm at room temperature was monitored for 20 min using a UV/Vis spectrophotometer. For all reactions 70 μm curcumin was added except for pH 9.0 where 50 μm curcumin was added. The buffers pH 5 to 8 were generated using mixtures of 200 mm Na2HPO4 and 100 mm citric acid, pH 8.5 and 9.0 were 100 mm K2HPO4 adjusted with NaOH. C, repetitive scans in 2-min intervals of a solution of 70 μm curcumin in 100 mm Tris-HCl buffer, pH 8.