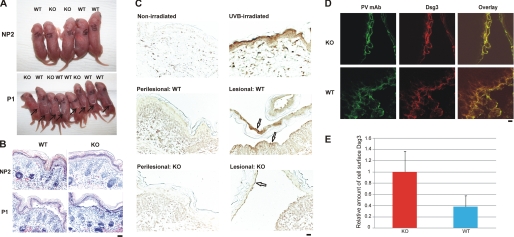
FIGURE 2.
Mice with a targeted deletion of p38α MAPK in the epidermis are susceptible to experimental PV. A, gross blister formation after passive transfer of pathogenic PV mAbs. p38 fl/fl;K14cre/+ (KO) and p38 fl/fl;+/+ (WT) mice were injected subcutaneously with 40 μg of nonpathogenic (NP2) or pathogenic (P1) PV IgG. Both WT and KO mice developed gross blisters (indicated by arrows) after P1 IgG passive transfer. B, P1 mAb causes suprabasal PV blisters in both WT and KO mice. Mice injected with nonpathogenic (NP2) PV mAb did not develop blisters. 12 of 13 WT mice and eight of eight KO mice injected with P1 mAb developed histologic blisters with suprabasal acantholysis typical of PV. Scale bar = 100 μm. C, p38 is activated in lesional but not perilesional keratinocytes of mouse epidermis after P1 mAb passive transfer. UVB irradiation led to phosphorylation of p38 in mouse epidermis. Activated p38 was markedly increased in lesional compared with perilesional keratinocytes from WT mice. Focal p38 activation was detected in lesional keratinocytes from p38α-deficient epidermis. Scale bar = 100 μm. D, confocal immunofluorescence microscopy of WT and KO mouse skin after pathogenic P1 mAb passive transfer. Localization of Dsg3 (green) and P1 IgG (red) is shown. Scale bar = 10 μm. E, quantification of cell surface Dsg3. The relative amount of cell surface Dsg3 was significantly increased in p38α-deficient skin keratinocytes compared with those in WT mice (p < 0.01 (Student's t test)). Confocal immunofluorescence experiments were performed three times; error bars indicate 1 S.D. the mean.