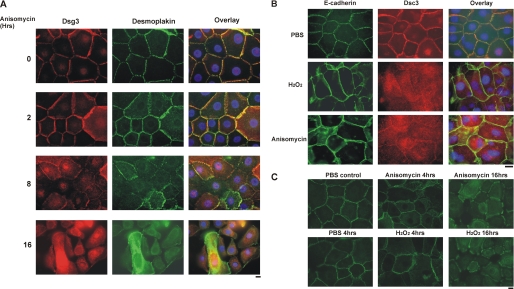
FIGURE 3.
Activation of p38 MAPK causes loss of desmosomal Dsg3. A, anisomycin disrupts the desmosomal cell surface localization of Dsg3. PHEK were treated with 0.4 mm calcium for 16 h to induce desmosome assembly, followed by treatment with the p38 activator anisomycin (100 μg/ml) for the specified amount of time. The localization of Dsg3 (red) or the desmosomal marker desmoplakin (green) was visualized by immunofluorescence microscopy. Nuclei (blue) were stained with DAPI, as shown in image overlay. Scale bar = 10 μm. B, desmosomal proteins are preferentially disrupted by p38 activation. PHEK were treated with 200 μm H2O2 or 200 μg/ml anisomycin for 4 h in medium containing 1.2 mm calcium. Localization of the adherens junction protein E-cadherin and the desmosomal cadherin desmocollin 3 was evaluated by immunofluorescence microscopy. Scale bar = 10 μm. C, activation of p38 causes internalization of cell surface Dsg3. PHEK were cultured in medium containing 0.4 mm calcium for 18 h to promote desmosome assembly. Cells were then incubated in the same medium containing a nonpathogenic anti-Dsg3 mAb tagged with GFP at 4 °C for 1 h to label cell surface Dsg3. After washing with PBS, cells were treated with 200 μm H2O2 or 100 μg/ml anisomycin for 0–16 h. Cells were fixed without permeabilization, and the localization of surface-labeled Dsg3 was evaluated by immunofluorescence microscopy. Scale bar = 10 μm.