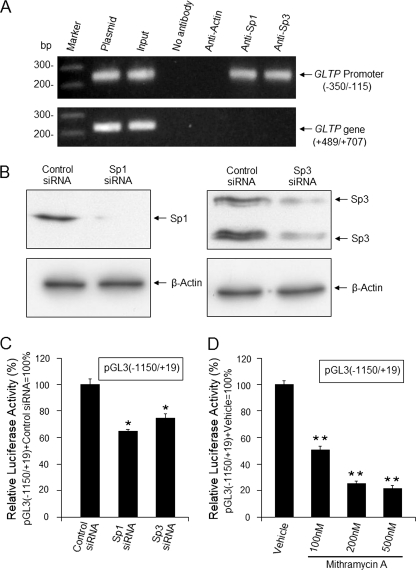
FIGURE 5.
Regulation of human GLTP promoter by Sp1 and Sp3 transcription factors. A, Sp1 and Sp3 binding to the GLTP promoter in vivo. ChIP assays were performed using DNA from HeLa cells as sources of the human GLTP promoter and specific antibodies for Sp1, Sp3, or β-actin. GLTP promoter region (−350/−115) containing putative Sp1/Sp3 binding sites was amplified by nested PCR as described under “Experimental Procedures.” The human GLTP exon 5 fragment (+489/+707), amplified using primer pair Ne-1/Ne-2, served as control lacking Sp1/Sp3 binding sites. Amplification controls were as follows: cross-linked, sheared DNA prior to immunoprecipitation (input); plasmid carrying (−350/−115) or (+489/+707) was used as template (plasmid). B and C, down-regulation of Sp1/Sp3 by siRNA knockdown reduces GLTP promoter activity. Cells transfected with pGL3(−1150/+19) were grown for 24 h, transfected with Sp1/Sp3 siRNA (25 nm), and grown for an additional 24 h before Western blot analysis and measurement of luciferase activity. D, mithramycin A treatment down-regulates Sp1/Sp3 expression and decreases GLTP promoter activity. Cells transfected with pGL3 (−1150/+19) were grown 24 h, treated with mithramycin A (0, 100, 200, or 500 nm) for 24 h, and analyzed for luciferase activity (normalized to Renilla luciferase activity). Vehicle, 0.1% DMSO. Bars show the means ± S.E. of three to six determinations in HeLa cells. *, p < 0.05; **, p < 0.01.