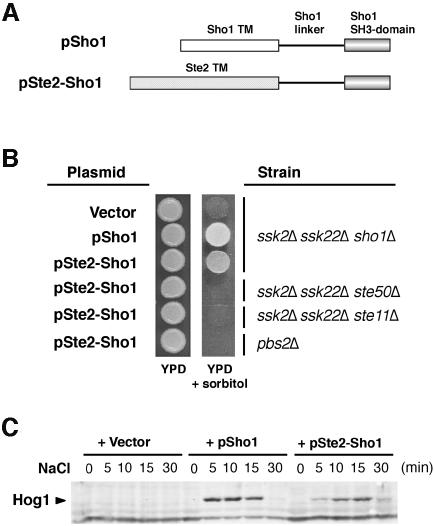
Fig. 2. A chimeric protein composed of the transmembrane domains of Ste2 and the cytoplasmic domain of Sho1 is functional. (A) Schematic representation of the wild-type Sho1 and the Ste2–Sho1 chimeric constructs. A region of the STE2 gene encoding the seven transmembrane (TM) domains was fused to a region of the SHO1 gene encoding the cytoplasmic linker region and the SH3 domain (residues 146–367). (B) Complementation of sho1Δ mutation by the Ste2–Sho1 chimera. Yeast strains MY007 (ssk2Δ ssk22Δ sho1Δ), FP67 (ssk2Δ ssk22Δ ste50Δ), FP50 (ssk2Δ ssk22Δ ste11Δ) and TM260 (pbs2Δ) were transformed with wild-type SHO1 (pSho1), the STE2–SHO1 chimeric construct (pSte2–Sho1), or the empty vector pYES2 (Vector). Rescue of the osmosensitive phenotype of this strain was assessed by spotting transformed cells onto YPD plates containing 1.5 M sorbitol. Although the Ste2–Sho1 chimeric protein could functionally substitute for wild-type Sho1, it failed to complement the osmosensitive defect of ste50Δ, ste11Δ and pbs2Δ strains (ssk2Δ and ssk22Δ mutations are included in some strains to inactivate the SLN1 branch of the HOG pathway). (C) The Ste2–Sho1 chimera can activate the Hog1 MAP kinase with kinetics similar to wild-type Sho1 protein in response to external osmotic stress. The same transformants as in (B) were tested by 4G10 immunoblot assay to detect phosphorylation of the Hog1 MAP kinase before (time 0), and 5, 10, 15 and 30 min after the addition of 0.4 M NaCl.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.