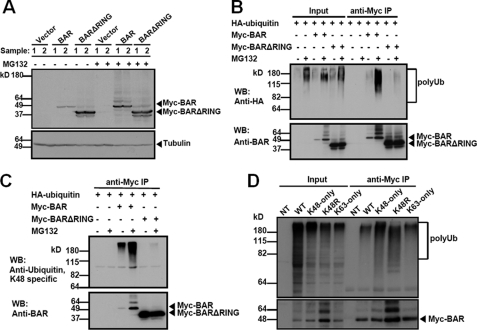
FIGURE 2.
BAR mediates RING-dependent self-ubiquitination and proteasomal degradation. A, 293T cells were singly transfected with pcDNA3 vector (first, second, seventh, and eighth lanes), or plasmids encoding Myc-BAR (third, fourth, ninth, and tenth lanes) or Myc-BARΔRING (fifth, sixth, eleventh, and twelfth lanes) for 24 h. 5 h before collecting the cells, proteasome inhibitor MG132 (20 μm) was added (+), as indicated. Cells lysates were analyzed by immunoblotting using rabbit anti-BAR antibody. α-Tubulin served as a loading control. Compared with BAR (third and fourth lanes), deletion of the RING domain stabilized the BAR protein level (fifth and sixth lanes). Treatment with MG132 also increased the BAR protein level (ninth and tenth lanes). B, 293T cells were co-transfected with HA-ubiquitin and pcDNA3 vector (first, second, seventh, and eighth lanes) or plasmids encoding BAR (third, fourth, ninth, and tenth lanes) or BARΔRING (fifth, sixth, eleventh, and twelfth lanes) for 24 h. MG132 was added (+) 5 h before collecting the cells, as indicated. Cell lysates were immunoprecipitated (IP) using mouse anti-Myc antibody. The inputs (1/20 of lysates used for immunoprecipitation) and the immunoprecipitated proteins were analyzed by immunoblotting using rabbit anti-HA to detect ubiquitination and anti-BAR antibody. Poly-Ub chains were detected in BAR immunoprecipitates (ninth lane), which were dramatically increased when cells were treated with MG132 (tenth lane). In contrast, deletion of the RING domain significantly reduced ubiquitination of BAR (eleventh and twelfth lanes). C, equivalent portions of the immunoprecipitated proteins described in B were analyzed by immunoblotting using rabbit anti-Lys48-specific ubiquitin antibody (upper) and anti-BAR antibody (lower). D, 293T cells were co-transfected with Myc-BAR and various HA-tagged ubiquitin plasmids (WT, K48-only, K48R, and K63-only) for 24 h. Cell lysates were immunoprecipitated with mouse anti-Myc antibody. Non-transfected cell lysate (NT) was used as a negative control for immunoprecipitation. The inputs (1/20 of lysates used for immunoprecipitation) and immunoprecipitates were analyzed by immunoblotting using rabbit anti-HA to detect ubiquitination and anti-BAR antibodies. Note that BAR was able to form poly-Ub chains joined through sites other than K48 (ninth and tenth lanes, upper panel).