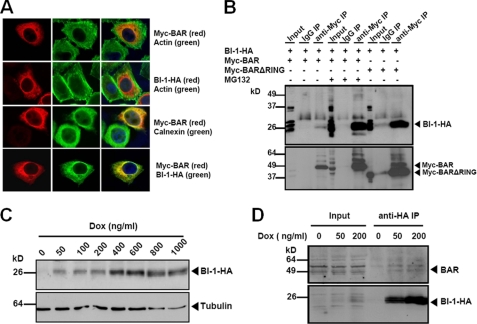
FIGURE 3.
BAR co-localizes and interacts with BI-1 in ER. A, HeLa cells were singly transfected with Myc-BAR, BI-1-HA, or co-transfected with both plasmids (normalizing total DNA transfected with pcDNA3). After 24 h, cells transfected with Myc-BAR were treated with 20 μm MG132 for 2 h. Cells were fixed and labeled with actin, Myc epitope, calnexin, or HA epitope antibodies as indicated, and analyzed by immunofluorescence confocal microscopy. B, 293T cells were co-transfected with BI-1-HA and Myc-BAR (first to sixth lanes) or Myc-BARΔRING (seventh to ninth lanes) for 48 h. MG132 was added 6 h before collecting cells, as indicated (fourth to sixth lanes). BAR or BARΔRING protein in cell lysates was immunoprecipitated with mouse anti-Myc antibody (third, sixth, and ninth lanes). Normal mouse IgG was used as a negative control for immunoprecipitation (second, fifth, and eighth lanes). The precipitates were analyzed by immunoblotting using rabbit anti-HA and anti-BAR antibodies. BI-1 co-immunoprecipitated with BAR (third lane), which was enhanced when treated with MG132 (sixth lane) or co-transfected with BARΔRING (ninth lane). C, inducible BI-1-HA-expressing HeLa cells were treated with doxycycline (Dox) at increasing concentrations for 24 h. Dose-dependent induction of BI-1-HA was detected by immunoblot. α-Tubulin served as a loading control. D, HeLa cells were induced to express BI-1-HA with low concentrations of Dox for 24 h. Cell lysates were immunoprecipitated with anti-HA antibody and subjected to immunoblotting with anti-BAR antibody. Cell lysates without Dox treatment were used as a negative control for immunoprecipitation (fourth lane). Note that BAR protein was detected in BI-1-HA immunoprecipitates (upper panel, fifth, and sixth lanes). The levels of precipitated BI-1-HA protein were also shown (lower panel).