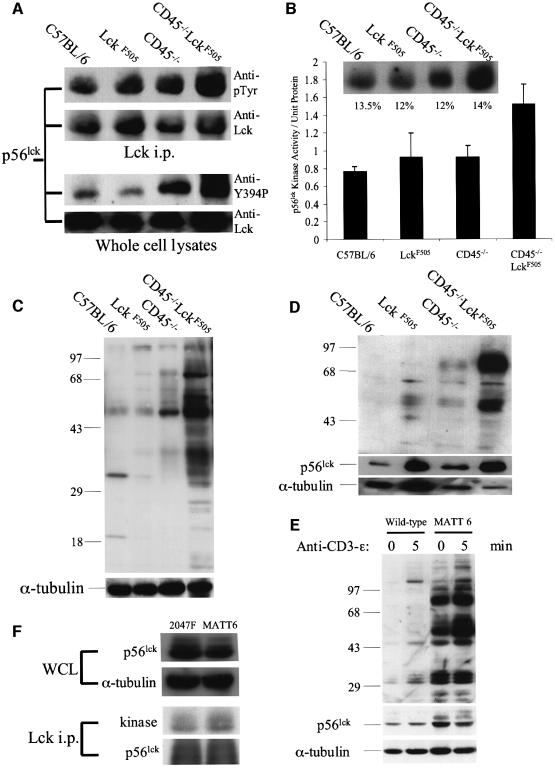
Fig. 6. Hyperactive p56lck kinase in CD45–/–LckF505 thymocytes induces increased protein tyrosine phosphorylation. All experiments were performed using pre-tumorigenic CD45–/–LckF505 thymocytes. (A) Immunoprecipitated p56lck was immunoblotted for phosphotyrosine (upper panels) and whole cell lysates were probed with an antibody recognizing p56lck pTyr394 (lower panels). Immunoblots were reprobed for p56lck to show comparable protein loading. (B) p56lck in vitro kinase activity is increased in CD45–/–LckF505 thymocytes. The insert illustrates phosphorylation levels in a representative experiment and the values shown are relative phosphorimager values obtained by reprobing for p56lck protein. The bar chart represents mean data ± SD from three independent experiments. Kinase values have been normalized for protein loading. The CD45–/–LckF505 values are significantly different (P <0.05) (Student’s t-test) from all other values shown. (C) Protein tyrosine phosphorylation is increased in CD45–/–LckF505 thymic lysates. The blot was reprobed for α-tubulin to show equal loading of protein. (D) The tyrosine phosphorylation of 56 and 80–85 kDa proteins in the detergent insoluble fraction of CD45–/–LckF505 thymocytes. The blot was reprobed for p56lck and for α-tubulin to show protein loading. Note that slight under-loading in the CD45–/–LckF505 track compared with C57BL/6 implies that both protein hyperphosphorylation and the p56lck protein level are greater than illustrated. See text for quantification of mean values. (E) The MATT-6 cell line established from a CD45–/–LckF505 tumour and wild-type thymocytes as control were stimulated with CD3-ε (2C11) mAb for the times indicated. The blot was reprobed for p56lck (middle panel), and for α-tubulin (bottom panel) to show comparable loading of protein. (F) Whole-cell lysates from MATT-6 (3 × 107) and 2047F (2 × 107) cells were probed for p56lck and α-tubulin (upper panels) and in vitro kinase assays were carried out in p56lck immunoprecipitates, which were subsequently probed for p56lck protein (lower panels). See text for quantification.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.