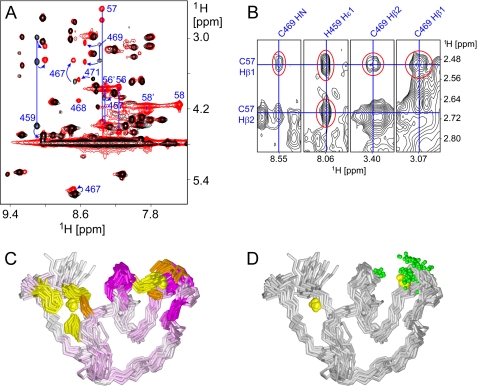
FIGURE 5.
Interaction of JUNV ZBD with acetyl-SCT. A, numerous crosspeak shifts are noticeable in the superimposed amide-aliphatic regions of two-dimensional TOCSY spectra of JUNV ZBD in the absence of acetyl-SCT (black) and JUNV ZBD in the presence of a 2-fold molar excess of acetyl-SCT (red). The most conspicuous crosspeak shifts are marked with blue arrows and residue numbers. Signals from both bound and free acetyl-SCT peptide are only present in the latter spectrum and are also labeled with residue numbers (56–58 and 56′-58′, respectively). Several blue lines that connect crosspeaks from the same residues are included for clarity. B, crosspeaks between JUNV ZBD and acetyl-SCT are observable in two-dimensional NOESY spectrum of JUNV ZBD in the presence of a 2-fold molar excess of acetyl-SCT. Several strips from the NOESY spectrum are shown with the intermolecular signals highlighted in red and chemical shifts that pass through the crosspeaks labeled in blue. C, superimposed backbone traces of JUNV ZBD are colored according to 1H chemical shift changes of individual residues upon binding of the acetyl-SCT peptide. HN chemical shifts were used where available; Hδ shifts were used for proline residues while Hα shifts were substituted for other residues without observable HN signals. Color intensity is proportional to the observed change. Zinc ions are depicted as yellow spheres, and cysteine and histidine side chains that coordinate the zinc ions are colored orange and yellow, respectively. Note that all significant chemical shift changes are localized to zinc cluster II, thus marking it as the acetyl-SCT-binding site. D, superimposed backbone traces of JUNV ZBD are shown with green spheres representing all atoms that exhibit unambiguous NOEs to the acetyl-SCT peptide. The orientation of the molecules is the same as in panel C. All atoms in JUNV ZBD for which unambiguous intermolecular NOEs were identified are localized at zinc cluster II.