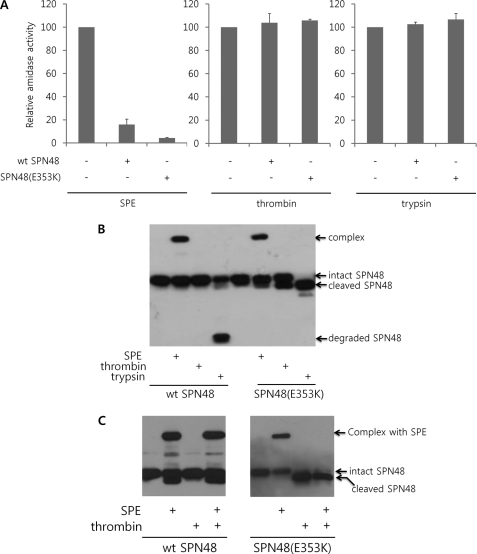
FIGURE 1.
Specific activity of SPN48. A, inhibitory effects of the wild-type and E353K SPN48 (400 ng each) on SPE, thrombin, and trypsin (100 ng each) were monitored by the amidase activity. The amidase activity of each protease was measured in the presence of wild-type SPN48 based on the fluorescence intensity liberated from the product. The peptide t-butyloxycarbonyl-Val-Pro-Arg-4-methylcoumaryl-7-amide was used as a substrate for SPE and thrombin, whereas the peptide t-butyloxycarbonyl-Phe-Ser-Arg-4-methylcoumaryl-7-amide was used for trypsin. Before measuring the amidase activities, the reaction mixtures were incubated for 4 h at 30 °C. Each experiment was performed in triplicate, and the standard deviations are shown as error bars. B, complex formation of wild-type and E353K SPN48 (400 ng each) with SPE (200 ng), thrombin (2 units), and trypsin (200 ng). The reaction mixtures were incubated for 30 min at 30 °C (for SPE) or 37 °C (for trypsin and thrombin) and then were subjected to SDS-PAGE and subsequent Western blotting using anti-SPN48 antibody. When SPN48 inactivates the target serine protease, a high molecular weight SDS-stable complex is formed with the target protease, as indicated. Alternatively, SPN48 is cleaved at the P1 residue without inactivating the target protease or is degraded, as indicated. C, comparison of wild-type SPN48 with the mutant SPN48 (E353K) in the specific inhibition of SPE in the presence of a nontargeting protease thrombin. Wild-type SPN48 (wt SPN48) forms a complex with SPE without being affected by thrombin, whereas thrombin severely interfered with the mutant SPN48 (E353K). The SPN48 was visualized by immunoblotting with anti-SPN48 antibody.