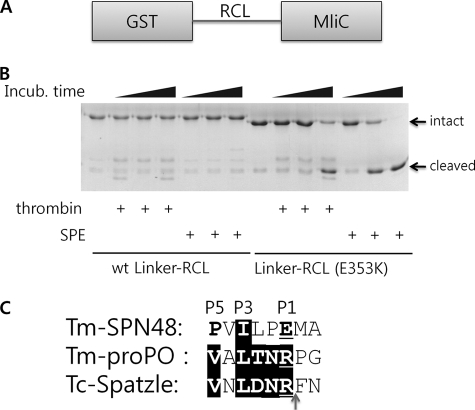
FIGURE 3.
Intrinsic flexibility of the SPN48 RCL region. A, domain structure of linker-RCLs. The RCL region of SPN48 is inserted in the GST and P. aeruginosa MliC proteins. B, protease activity of SPE and thrombin on wild-type and mutant linker-RCLs. Wild-type or mutant linker-RCL was used as a substrate for thrombin or SPE for the specified time at 30 °C. Although the wild-type protein (wt Linker-RCL) remained uncleaved, the mutant protein (Linker-RCL (E353K)) was gradually degraded by both proteases. C, sequence alignment of SPN48 RCL and the SPE substrates T. molitor prophenol oxidase (Tm-pro-PO) and Spätzle around the cleavage sites, indicated by an arrow. Because T. molitor Spätzle has not been cloned, its homologue Tribolium castaneum Spatzle (Tc-Spatzle) was used instead.