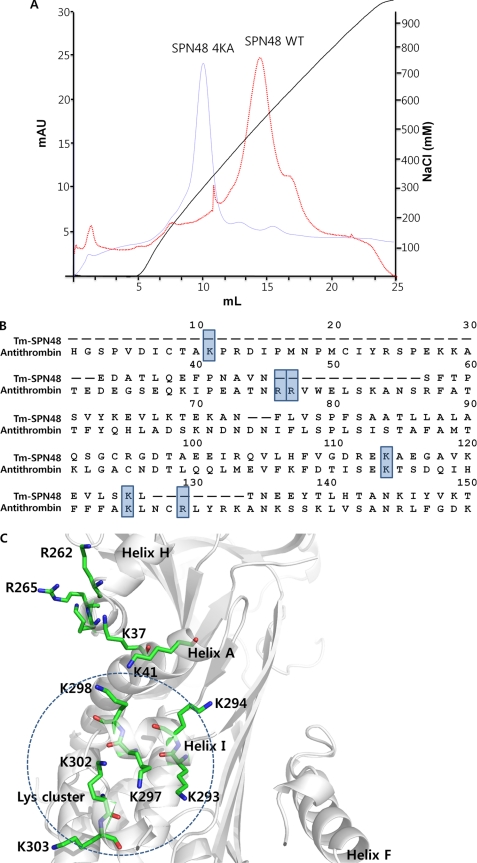
FIGURE 4.
Heparin-binding site of SPN48. A, heparin binding capacity of wild-type and mutant SPN48. The heparin binding activity of wild-type and mutant SPN48 4KA (K293A/K294A/K297A/K298A) was assessed by heparin-affinity chromatography. The SPN48 protein peak was confirmed by Western blotting (data not shown). B, sequence alignment of SPN48 with antithrombin, focusing on the residues at the classical heparin-binding site in antithrombin. The lysine or arginine residues involved in heparin binding are indicated by a shaded box (33, 35). Of six residues, only two residues are conserved in SPN48. C, putative heparin binding region of SPN48. All lysine or arginine residues of SPN48 are displayed with secondary elements shown in gray. Helix I, which is located on a surface opposite Helix D, contains Lys-293, Lys-294, Lys-297, and Lys-298, indicated by a dotted circle.