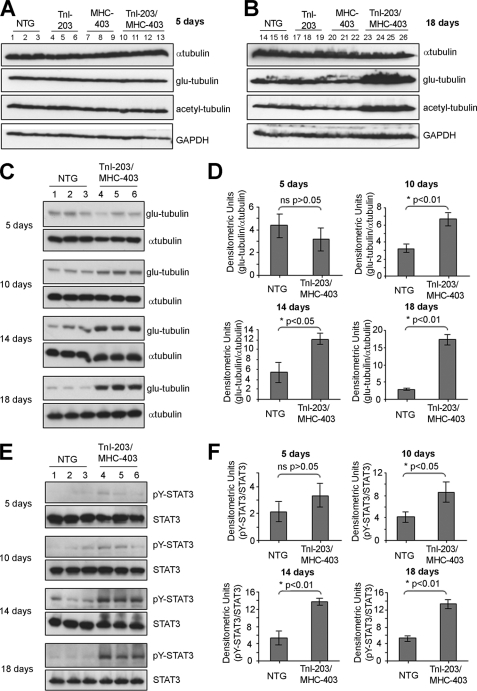
FIGURE 2.
Increases in cardiac glu-tubulin levels in TnI-203/MHC-403 mice coincide with elevated STAT3 phosphorylation. Protein extracts from the hearts of 5-day-old (A) or 18-day-old (B) non-transgenic (NTG, n = 3), TnI-203 (n = 3), MHC-403 (n = 3), and double mutant TnI-203/MHC-403 mice (n = 4) were immunoblotted for α-tubulin as an indicator of the total MT network and post-translationally modified glu- and acetyl-tubulin as indicators of MT stabilization. Immunoblotting for GAPDH indicated equivalent protein loading in all samples. Protein extracts from the hearts of 5, 10, 14, and 18 day old NTG and TnI-203/MHC-403 mice were immunoblotted for glu-tubulin, α-tubulin (C), Tyr-705-phosphorylated STAT3 and STAT3 (E). Glu-tubulin (D) and pY705-STAT3 (F) bands were quantified by densitometric analysis, normalized for α-tubulin and total STAT3 levels, respectively, and values expressed as mean ± S.D. (n = 3).