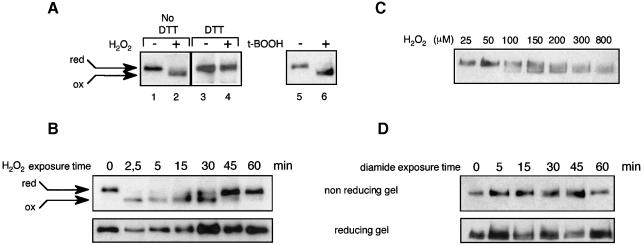
Fig. 2. In vivo oxidation of Yap1. (A) yMyc-Yap1 cultures (grown to an OD600 of 0.4) before and after 2.5 min treatment with 400 µM H2O2 (lanes 1 and 2) or 1 mM t-butyl hydroperoxide (lanes 5 and 6) were lysed using TCA, treated with iodoacetamide and immunoblotted after non-reducing SDS–PAGE. Lanes 3 and 4 are the same as lanes 1 and 2 except that the TCA-precipitated protein pellet was dissolved in the presence of 200 mM DTT, before adding a 3.2 M excess of iodoacetamide. (B) Time course of Yap1 oxidation by H2O2. A yMyc-Yap1 culture before and 2.5, 5, 15, 30, 45 and 60 min after treatment with 400 µM H2O2, processed as in (A) (lanes 1 and 2), except that Yap1 was dephosphorylated and separated under non-reducing or reducing conditions as indicated. (C) The minimum H2O2 concentration required to oxidize Yap1 in vivo. A yMyc-Yap1 culture treated for 5 min with 25, 50, 100, 150, 200, 300 and 800 µM H2O2 and processed as in (A) (lanes 1 and 2). (D) Time-course analysis of Yap1 redox status in response to diamide. A yMyc-Yap1 culture before and 5, 15, 30, 45 and 60 min after treatment with 2 mM diamide, processed as in (A) (lanes 1 and 2) and separated under non-reducing or reducing conditions as indicated.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.