Figure 1.
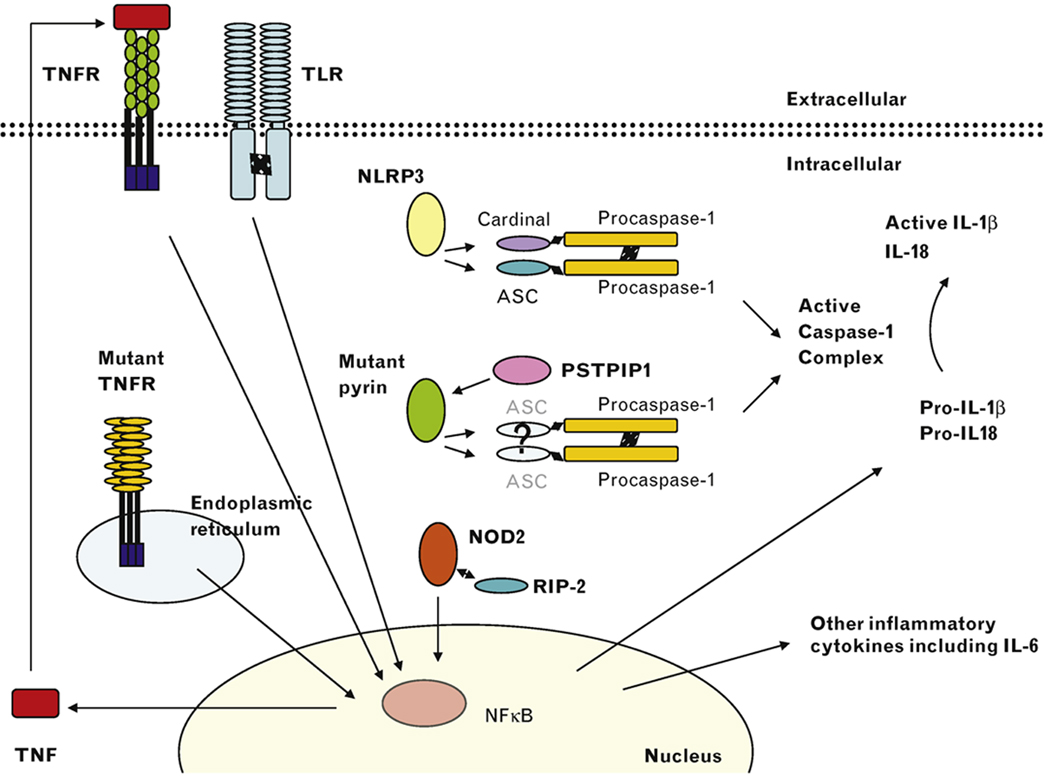
CAPS, FMF, and PAPA result in activation of the caspase-1 complex. In CAPS, mutations in NLRP3 result in increased assembly of the NLRP3 inflammasome and active caspase-1 through interactions with cardinal, ASC, and procaspase-1. In PAPA, mutations in PSTPIP1 result in its increased binding to pyrin and activation of active caspase-1. In FMF, mutant pyrin is pro-inflammatory in some conditions and results in caspase-1 activation. Active caspase-1 then cleaves pro-IL-1β to form active IL-1β and IL-18 in CAPS, FMF, and PAPA. In PGA, mutant NOD2 binds RIP-2 leading to increased NF-κB production and the transcription of pro-IL-1β and other inflammatory cytokines. In TRAPS, the mutant TNF receptor (TNFR) is sequestered in the endoplasmic reticulum leading to NF-κB production and transcription of a number of pro-inflammatory cytokines. An inflammatory feedback loop is generated through the production of TNF-α that can bind and signal through the wild-type TNFR on the cell surface. Toll-like receptor (TLR) stimulation in vitro induces pro-IL-1β and other cytokine production and models an essential first step in initiating an inflammatory response.
