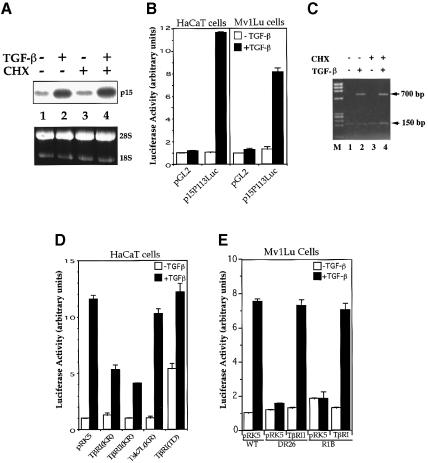
Fig. 1. TGF-β-induced p15Ink4B transcription requires functional TβRI and TβRII and is independent of de novo protein synthesis. (A) p15Ink4B expression is an immediate early response gene to TGF-β. Exponentially growing HaCaT cells were treated with or without 400 pM TGF-β or 10 µM cycloheximide (CHX). p15Ink4B mRNA was detected by northern hybridization. Equal levels of RNA were loaded per lane, as illustrated by equal levels of 28S and 18S RNA. (B) Transcriptional activation from the p15Ink4B promoter is induced by TGF-β in HaCaT and Mv1Lu cells. Cells were transfected with the p15P113luc luciferase reporter plasmid and, 40–45 h after transfection, treated with TGF-β for 4 h, and luciferase values were measured. (C) Luciferase mRNA expression from the p15Ink4B promoter in response to TGF-β does not require new protein synthesis. HaCaT cells were transfected with the p15P113luc luciferase reporter plasmid and a control β-galactosidase expression plasmid pSVβgal, incubated with TGF-β and/or cycloheximide for 4 h as shown, and RNA was isolated. The levels of luciferase and β-galactosidase mRNA were assessed by PCR cDNA amplification. While the 150 bp β-galactosidase cDNA band was constant in all lanes, the 700 bp luciferase cDNA band was induced by TGF-β, both in the absence and presence of cycloheximide (CHX). M, DNA fragment length markers (ΦX174 DNA/HaeIII). (D) Dominant-negative inhibition of TGF-β-induced p15Ink4B transcription by kinase-inactive (KR) TβRI and TβRII. HaCaT cells were cotransfected with p15P113luc and the indicated receptor expression plasmids. (E) TGF-β-induced p15Ink4B transcription requires both TβRII and TβRI. The reporter plasmid p15P113luc was transfected into wild-type Mv1Lu cells, which express both receptors, or the derivative DR26 and R1B cells, which lack functional TβRII and TβRI, respectively. Expression plasmids for TβRII or TβRI were cotransfected, as marked. All assays were done in triplicate and all values were normalized for transfection efficiency against the β-galactosidase expression directed from the cotransfected pSV-β-Gal control plasmid.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.