Abstract
The group II metabotropic glutamate receptors 2 and 3 (mGluR2 and mGluR3) share sequence homology, common pharmacology and negative coupling to cAMP. We recently discovered that mGluR3 also is negatively coupled through a G-protein to the cGMP transduction pathway in rat cerebellar granule cells and astrocytes. To test the hypothesis that mGluR2 also has access to the cGMP pathway, C6 glioma cells were stably transfected with mGluR2 and mGluR3 cDNA and their coupling to cGMP levels was characterized. In contrast to many other cell lines, C6 has a robust cGMP response that makes it attractive in the study of receptor coupling to this second messenger pathway. Consistent with prior studies, the mGluR3 receptor was negatively coupled to cGMP and this coupling was blocked by PTX. In contrast, mGluR2 agonists failed to reduce sodium nitroprusside stimulated cGMP levels in transfected cell lines where the receptor was negatively coupled to cAMP. These data provide further support for the functional divergence between these two closely related receptors.
Keywords: Group II metabotropic receptors, mGluR2, cGMP
Introduction
The actions of L-glutamate in the brain are mediated by ionotropic and metabotropic glutamate receptors that are present on neurons and astrocytes. Metabotropic glutamate receptors are classified into three groups based on their sequence similarity, pharmacological properties and signal transduction mechanisms (reviewed in: Conn 2003, Nicoletti et al., 2007). mGluR2 and mGluR3 constitute the group II metabotropic receptors. High affinity agonists and antagonists have been developed that activate or block group II receptors but most fail to effectively discriminate between mGluR2 and mGluR3 (Johnson et al., 1999; Conn, 2003; Nicoletti et al., 2007).
With a few exceptions (Wroblewska et al., 1993, Wroblewska et al., 1998), (Aronica et al., 1993, Schoepp et al., 1997), most direct studies of the coupling of group II mGluRs to second messenger cascades have been performed in continuous cell lines (Wroblewska et al., 1997). This is due to the difficulty in obtaining a homogeneous population of primary cells that selectively express mGluR2 or mGluR3. However, most of the cell lines that were used for these studies lack a robust cGMP response while having an active cAMP pathway. As a result, no studies using these cell lines have reported testing the potential coupling of group II receptors to a cGMP cascade. In contrast, cerebellar granule cell neurons and cerebellar astrocytes in culture expressed high levels of guanylate cyclase and mGluR3. We used these cells to demonstrate that mGluR3 was negatively coupled to both cAMP and cGMP (Wroblewska et al., 1997, Wroblewska et al., 2006). The aim of the present study was to test the hypothesis that mGluR2 receptors also can be coupled to cGMP via a G-protein. Given the difficulty in testing this hypothesis in a mixed population of cells that may express both mGluR2 and mGluR3 and the low levels of expression of guanylate cyclase in many cell lines, we elected to study this question in C6 glioma cells that had been stably transfected with mGluR2 and mGluR3 cDNA.
Materials and Methods
Drugs
The group II agonist, LY354740 was a generous gift from Eli Lilly & Co. The group II agonist DCGIV (Cartmell et al., 1998) and NAAG were purchased from TocrisTM. Before experiments, NAAG was repurified to remove glutamate contamination using AG 50W-X8 cation exchange (Bio-Rad). Sodium nitroprusside (SNP, an activator of guanylate cyclase), IBMX (iso-buthyl-methyl-xanthine, an inhibitor of phosphodiesterases) were from Sigma-Aldrich and Forskolin (an activator of adenylate cyclase) was from Calbiochem. Pertussis toxin (Bordatella pertussis, PTX) was purchased from List Biological Laboratories Inc.
Transfected C6 cells
The C6 cell line was obtained from American Tissue Culture Collection (ATCC CCL-107). Cells were grown in Ham F10 medium supplemented with 15% horse serum, 12.5% FBS, and gentamicin (50μg/ml). C6 cell lines stably expressing mGluR3 and mGluR2 receptor were prepared as described previously (Wroblewska et al., 1997). using rat mGluR2 and mGluR3 cDNA, a generous gift from Dr. Shigetada Nakanishi, at the Department of Molecular and System Biology, Graduate School of Biostudies, Kyoto University, Kyoto 606–8501, Japan. Briefly, C6 glioma cells were transfected using the calcium phosphate method (Invitrogen) and positive clones were selected with Geneticin (G-480, Gibco). The expression of mRNA in cell lines was confirmed using RT-PCR with mGluR2 and mGluR3 specific primers (Santi et al., 1994).
Cell treatments and cyclic nucleotides determination
The procedure for cell treatment and determination of cAMP and cGMP levels was as described previously (Wroblewska et al., 2006). Cell lines were grown to confluency before changes in cyclic nucleotides levels were determined in response to receptor activation. All experiments using the transfected cell lines were conducted in the presence of 300 μM IBMX to inhibit phosphodiesterases. Levels of cGMP and cAMP were detected in the cell extracts from individual culture wells using acetylated an I125-cGMP and I125-cAMP kits (Amersham). In each individual experiment on cells grown in culture and tested at the same time, three cell cultures were treated with control medium or with each of the drug treatments.
rtPCR
Total RNA was extracted from cells by the guanidinium thiocyanate method (TRI Reagent, Sigma-Aldrich). RT reaction was conducted using M-MLV reverse transcriptase (Invitrogen) and random hexamers as primers. Primers for PCR amplification of mGluR2 were: 5′-AAGTGCCCGGAGAACTTCAACGAA-3′ and 5′-AAGGCGACGACGTTGTTGAGTCCA-3′. These primer’s sequences were checked against mGluR3 sequence and found to have the least possible homology. No amplification of mGluR3 was detected when these primers were tested against mGluR3 transfected cells.
Western blots
Membranes were isolated from C6, C6mGluR2 and C6 mGluR3 cells grown to confluency. Cells were washed with 10 ml PBS pH 7.5 and 10 ml Tris 50 mM, pH 7.5. Samples were frozen at −80°C, sonicated, sedimented at 12,000 × g, washed several times and resuspended in 50 mM Tris pH 7.5. Membrane protein was quantified using Pearce standard kit. For Western blots, membrane protein (15 μg) was incubated for 60 min with equal volume of denaturing buffer (BioRad) supplemented with 300 mM DTT. Samples were loaded on an 8/16% Tris glycine Laemmli’s gel (Invitrogen). Protein bands were transferred to nitrocellulose membranes (Invitrogen) according to the manufacturer instructions. mGluR2/3 polyclonal antibody from Chemicon (AB1553). The deduced non-glycosylated size of the mGluR2 and mGluR3 receptor monomers is in the 99,000 kD range, corresponding to the glycosylated 103–106 kD bands on Western blots. Under these electrophoresis conditions, these mGluR2 receptors tend to migrate predominately as dimmers and monomers while mGluR3 migrates mostly as a monomer. Membranes were incubated for one hour with primary antibodies followed by an hour with donkey anti-rabbit peroxidase linked secondary antibody (Amersham). The ECL technique (Amersham) was used for the detection of immunoreactive protein on the blot.
Data Analysis
Cyclic nucleotide levels (pg/ml) were determined for each cell culture extract relative to values obtained from SNP or forskolin treated cultures in that experiment (cells inoculated into culture wells at the same time). The resulting percent values from individual cell cultures given the same treatment across experiments were summed for statistical analysis. As indicated in the figure legends, cyclic nucleotide levels are expressed as a percentage of the stimulation resulting from treatment with sodium nitroprusside (cGMP) or forskolin (cAMP). Data are expressed as means ± SEM. Student’s t-test was used to determine statistical significance between treatment groups where the value of “n” is the number of cultures that shared a treatment.
Results
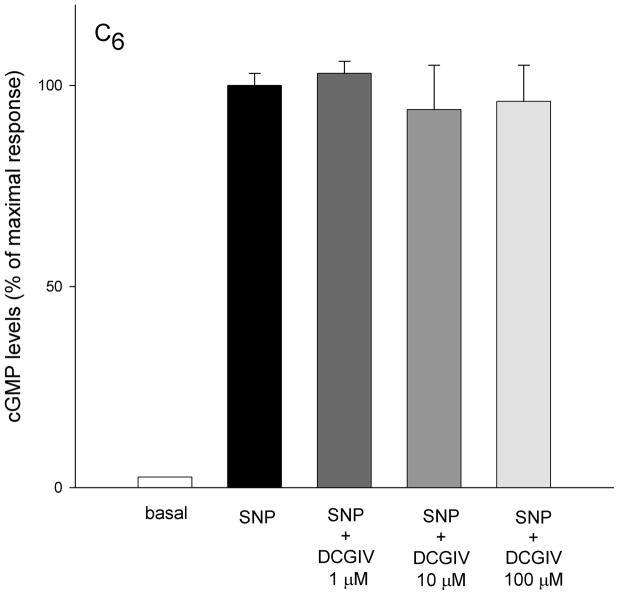
In the non-transfected C6 glioma cell line, sodium nitroprusside (100 μM SNP) significantly increased cGMP (18 ± 0.4 to 704 ± 21.2 pg/ml) in the presence of phosphodiesterase inhibitor (IBMX, 300 μM) (Figure 1). In order to determine if this glioma cell line expressed a significant level of group II receptors negatively coupled to cGMP, cells were treated with SNP followed by the group II selective agonist DCGIV (1, 10, 100 μM). DCGIV did not affect the SNP-induced cGMP levels.
Figure 1.
In cultures of a non-transfected C6 glioma cell line, application of sodium nitroprusside (SNP 100 μM) significantly increased cGMP formation. Basal levels of cGMP in C6 glioma cell line were stimulated almost 40-fold with 100 μM sodium nitroprusside (18 ± 0.4 to 704 ± 21.2 pg/ml, respectively). The group II mGluR agonist DCGIV (1, 10 100 μM) did not significantly change sodium nitroprusside-stimulated cGMP formation. Cells were grown on 96 wells, washed twice with Locke buffer, preincubated for 10 min at room temperature with the buffer containing phosphodiesterase inhibitor (300 mM IBMX) and incubated with agonists for 6 min. Results are expressed as means ± SEM (n = 6 cultures per treatment group). Statistical significance was determined by Student’s t-test in all figures. *P<0.05, **p<0.01
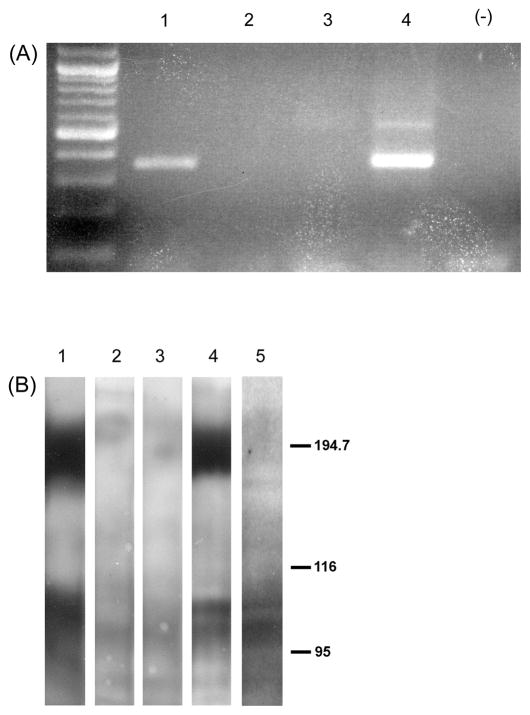
As previously reported (Yao et al., 2005), rtPCR amplification showed no evidence of mGluR2 mRNA in C6 cells prior to transfection. In contrast, the transfected cells give a strong PCR signal for mGluR2 message (Figure 2A). The expression of mGluR2 and mGluR3 receptor protein in the transfected cell lines was confirmed by Western blot (Figure 2b).
Figure 2.
A. rtPCR analysis of transcripts from non-transfected C6 cells (lanes 2 and 3) and two C6 cells stably transfected with mGluR2 (lanes 1 and 4). Major DNA bands in lanes 1 and 4 are in the size range predicted for mGluR2 rtPCR products obtained via the primers used in this study.
B. Western blot analysis of protein from non-transfected C6 cells (lanes 2 and 3), two C6 cell lines stably transfected with mGluR2 (lanes 1 and 4) and one C6 cell line stably transfected with mGluR3 (lane 5). Blots were reacted with mGluR2/3 antibody (Chemicon). Under these experimental conditions, mGluR2 receptors migrate more as dimers (~200 kD) than monomers (~100 kD) while mGluR3 tends to migrate primarily as the monomer (~100 kD).
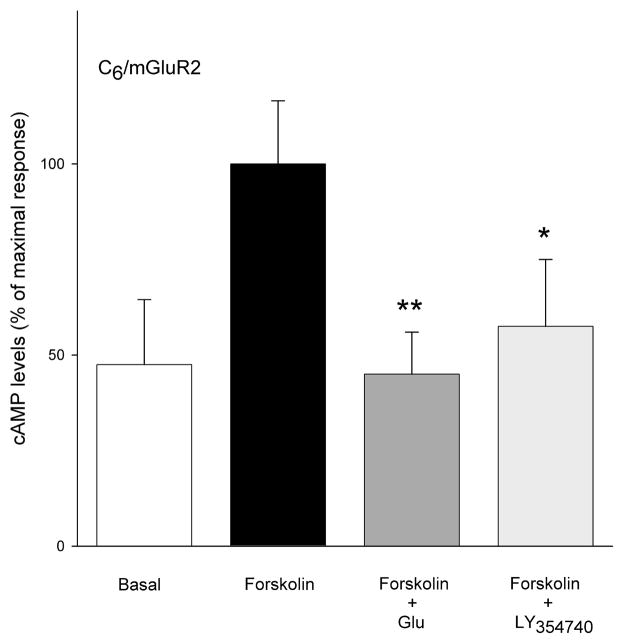
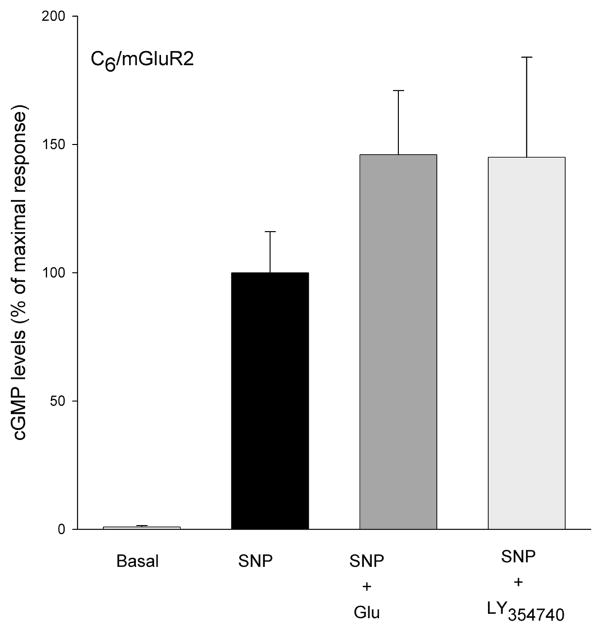
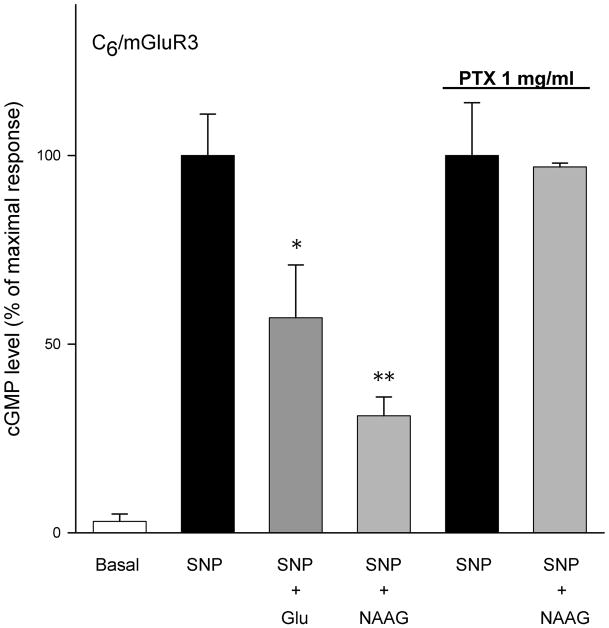
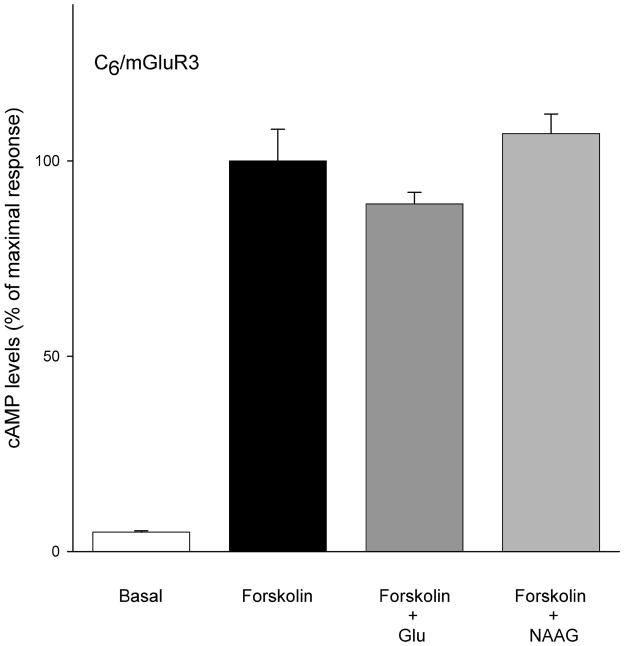
Group II agonists L-glutamate (100 μM) and LY354740 (10 μM) significantly decreased forskolin stimulated cAMP levels in the cell lines that were stably transfected with mGluR2 (Figure 3). In the same cell line, neither LY35740 (10 μM) nor L-glutamate (100 μM) significantly affected sodium nitroprusside-stimulated cGMP formation (Figure 4A). This contrasts the efficacy of L-glutamate (100 μM) and re-purified NAAG (100 μM) in reducing cGMP levels in C6 cells stably transfected with mGluR3 (Figure 4B). Coupling of mGluR3 to cGMP in these cells was PTX sensitive (Figure 4B). However, both L-glutamate and NAAG, failed to reduce forskolin-stimulated cAMP formation in C6/mGluR3 cells (Figure 5).
Figure 3.
C6 cells stably expressing mGluR2 receptors were stimulated with forskolin (10 μM) to increase levels of cAMP in the presence of phosphodiesterase inhibitor IBMX. The group II agonists L-glutamate at 100 μM and LY354740 at 10 μM significantly reduced these levels. Results are expressed as mean ± SEM and represent two separate clones of C6mGluR2 cell lines (n = 9)
Figure 4.
(A) C6/mGluR2 cells were treated with SNP (100 μM) to stimulate cGMP formation and IBMX (300 mM) to inhibit phosphodiesterase activity. The group II agonists L-glutamate (100 μM) and LY354740 (10 μM) failed to decrease SNP stimulated cGMP levels. Results are mean ± SEM from four separate transfected cell lines, each assayed in triplicate cultures (n = 12).
(B) cGMP levels in four lines of C6 cells stably transfected with mGluR3 were stimulated with SNP (100μM). NAAG (100 μM) and L-glutamate (100 μM) reduced cGMP levels in these cells. (p>0.05, n = 24 cultures). Overnight incubation with 1 μg/ml of PTX abolished this inhibition.
Figure 5.
In C6/mGluR3 cells, both L-glutamate (100 μM) and NAAG (100 μM) did not significantly change forskolin stimulated cAMP response. Results are expressed as mean ± SEM (n = 12).
Discussion
The G-protein coupled receptors (GPCR) are a large and diverse superfamily of receptors to which the mGluRs belong (Flower 1999). GPCRs transduce a wide range of extracellular signals into cellular responses. The common characteristic of GPCR is seven transmembrane alpha-helices, while the C- and N-terminuses of these receptors differ markedly. As a result of variation in the cytoplasmic loops connecting these transmembrane helices, the G-protein coupling among them is diverse. It has long been recognized that mGluRs are linked to either PI hydrolysis or reduction in cAMP levels (Pin and Duvoisin 1995). In the absence of other data, it had been assumed that these were the only second messenger pathways directly regulated by mGluRs. Consistent with this view, we previously reported that group II mGluRs were negatively coupled to cAMP levels in cerebellar granule cell neurons and astrocytes (Wroblewska et al. 1998). More recently, we found that group II receptors in these cells also are negatively coupled to a cGMP transduction system (Wroblewska et al., 2006). Based on the sensitivity of this receptor to NAAG and FN6, together with the preponderance of mGluR3 expression in these cells, we concluded that this receptor was mediating the cGMP response.
Based on these data and given the structural homology between mGluR2 and mGluR3, we speculated that mGluR2 also negatively coupled via a G protein to cGMP levels. In the course of examining this question, we tested cell lines that we and others commonly use for these transfection studies and found that none had high levels of guanylate cyclase responding to SNP stimulation. In contrast, C6 glioma cells expressed substantial levels of this enzyme activity (Figure 1).
Militating against our initial speculation, C6 cells stably expressing mGluR2 did not exhibit significant coupling to the cGMP mediated signal transduction (Figure 4A). In the absence of this response, one might conclude that the transfected mGluR2 cDNA simply failed to be expressed in a form that permitted G protein coupling in C6 cells. Refuting this, mGluR2 was negatively coupled to cAMP levels in these (Figure 3), a result that supports our previous study on mGluR2 expression in CHO cells (Wroblewska et al., 1997).
One interpretation of these data is that C6 cells fail to express the G proteins required to couple group II receptors to the cGMP pathway. However in a parallel study, C6 cells were stably transfected with mGluR3. When these cells were challenged with 100 uM glutamate or repurified NAAG, SNP-stimulated levels of cGMP were significantly reduced (Figure 4B). This result is consistent with data obtained in primary neurons and astrocytes (Wroblewska et al., 2006).
We previously produced several CHO cell lines stably transfected with mGluR3 (Wroblewska et al., 1997) but these cell lines quickly lost the negative coupling of the receptor to cAMP levels although receptor message continued to be expressed (unpublished observations). This was in contrast to CHO cell lines stably transfected with mGluR1, 2, 4 and 5. This suggested an uncoupling of the mGluR3 to adenylate cyclase or a failure of cells that retain this coupling to efficiently divide in standard culture medium with serum. The cAMP levels in C6 glioma cells that were stably transfected with mGluR3 in the present study also were stimulated with forskolin. Treatment of these C6 mGluR3 cells with glutamate (100 μM) or NAAG (100 μM) failed to significantly reduce cAMP (Figure 5) while maintaining negative coupling to cGMP (Figure 4B). This result supports the view that CHO and C6 cell lines that are stably transfected with mGluR3 an uncoupling occurs with the cAMP cascade while the receptor continues to regulate levels of cGMP.
It has been shown previously that individual neurotransmitter receptors may couple to more than one G-protein (Albert and Robillard 2002). This is true for both Gi and Go/s proteins (Heubach et al., 2004). Beyond this, it has been well documented that different ligands may activate different g-protein pathways at the same receptor (reviewed in Ambrosio et al., 2011). Indeed, the efficacy of glutamate, but not NAAG, in the activation of a G-protein coupled potassium channel in cells transiently transfected with mGluR3 likely represents this phenomenon (Chopra et al., 2009; Fricker et al., 2009). The failure of mGluR2 to couple to cGMP while successfully coupling to the cAMP pathway supports the hypothesis that mGluR2 lacks the ability to regulate cGMP levels. This conclusion awaits testing in primary neurons. If confirmed, this differential coupling of mGluR2 and mGuR3 with the cGMP cascade may have considerable significance as drugs that discriminate between these receptors, such as mGluR2 positive allosteric modulators, are developed for clinical use (Conn et al. 2009, Fraley 2009, Jin et al. 2010).
Research Highlights.
Metabotropic glutamate receptor 3 negatively couples to cyclic GMP cascade
Metabotropic glutamate receptor 2 does not couple to cGMP cascade
Drugs that discriminate mGluR2 and mGluR3 will have different clinical applications
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Albert PR, Robillard L. G protein specificity: traffic direction required. Cell Signal. 2002;14:407–418. doi: 10.1016/s0898-6568(01)00259-5. [DOI] [PubMed] [Google Scholar]
- Ambrosio M, Zurn A, Lohse M. Sensing G protein-coupled protein activation. Neuropharmacology. 2011;60:45–51. doi: 10.1016/j.neuropharm.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Aronica E, Nicoletti F, Condorelli DF, Balazs R. Pharmacological characterization of metabotropic glutamate receptors in cultured cerebellar granule cells. Neurochem Res. 1993;18:605–12. doi: 10.1007/BF00966938. [DOI] [PubMed] [Google Scholar]
- Cartmell J, Adam G, Chaboz S, Henningsen R, Kemp JA, Klingelschmidt A, Metzler V, Monsma F, Schaffhauser H, Wichmann J, Mutel V. Charaterization of [3H]-(2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine ([3H]-DCG IV) binding to metabotropic mGlu2 receptor-transfected cell membranes. Brit J Pharm. 1998;123:497–504. doi: 10.1038/sj.bjp.0701647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra M, Yao Y, Blake TJ, Hampson DR, Johnson EC. The neuroactive peptide N-acetylaspartylgluatamate Is not an agonist at the metabotropic glutamate receptor subtype 3 of metabotropic glutamate receptor. J Pharmacol Exp Ther. 2009;330:212–219. doi: 10.1124/jpet.109.152553. [DOI] [PubMed] [Google Scholar]
- Conn PJ. Physiological roles and therapeutic potential of metabotropic glutamate receptors. Ann N Y Acad Sci. 2003;1003:12–21. doi: 10.1196/annals.1300.002. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Jones CJ. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraley ME. Positive allosteric modulators of the metabotropic glutamate receptor 2 for the treatment of schizophrenia. Expert Opin Ther Pat. 2009;19:1259–75. doi: 10.1517/13543770903045009. [DOI] [PubMed] [Google Scholar]
- Fricker AC, Mok MH, de la Flor R, Shah AJ, Woolley M, Dawson LA, Kew JN. Effects of N-acetylaspartylglutamate (NAAG) at group II mGluRs and NMDA. Neuropharmacology. 2009;56:1060–7. doi: 10.1016/j.neuropharm.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Flower DR. Modelling G-protein-coupled receptors for drug design. Biochim Biophys Acta. 1999;1422:207–34. doi: 10.1016/s0304-4157(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Heubach JF, Ravens U, Kaumann AJ. Epinephrine Activates Both Gs and Gi Pathways, but Norepinephrine Activates Only the Gs Pathway through Human {beta}2-Adrenoceptors Overexpressed in Mouse Heart. Mol Pharmacol. 2004;65:1313–1322. doi: 10.1124/mol.65.5.1313. [DOI] [PubMed] [Google Scholar]
- Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, Conn PJ, Cosford ND, Markou A. The mGluR2 Positive Allosteric Modulator BINA Decreases Cocaine Self-Administration and Cue-Induced Cocaine-Seeking and Counteracts Cocaine-Induced Enhancement of Brain Reward Function in Rats. Neuropsychopharmacology. 2010 Jun 16;2010 doi: 10.1038/npp.2010.82. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BG, Wright RA, Arnold MB, Wheeler WJ, Ornstein PL, Schoepp DD. [3H]- LY341495 as a novel antagonist radioligand for group II metabotropic glutamate (mGlu) receptors: characterization of binding to membranes of mGlu receptor subtype expressing cells. Neuropharmacology. 1999;38:1519–29. doi: 10.1016/s0028-3908(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Lea PM, 4th, Wroblewska B, Sarvey JM, Neale JH. beta-NAAG rescues LTP from blockade by NAAG in rat dentate gyrus via the type 3 metabotropic glutamate receptor. J Neurophysiol. 2001;85:1097–1106. doi: 10.1152/jn.2001.85.3.1097. [DOI] [PubMed] [Google Scholar]
- Nicoletti F, Battaglia G, Storto M, Ngomba RT, Iacovelli L, Arcella A, Gradini R, Sale P, Rampello L, De Vita T, Di Marco R, Melchiorri D, Bruno V. Metabotropic glutamate receptors: beyond the regulation of synaptic transmission. Psychoneuroendocrinology. 2007;32(Suppl 1):S40–5. doi: 10.1016/j.psyneuen.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Santi MR, Ikonomovic S, Wroblewski JT, Grayson DR. Temporal and depolarization-induced changes in the absolute amounts of mRNAs encoding metabotropic glutamate receptors in cerebellar granule neurons in vitro. J Neurochem. 1994;63:1207–17. doi: 10.1046/j.1471-4159.1994.63041207.x. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Johnson BG, Wright RA, Salhoff CR, Mayne NG, Wu S, Cockerham SL, Burnett JP, Belegaje R, Bleakman D, Monn JA. LY354740 is a potent and highly selective group II metabotropic glutamate receptor agonist in cells expressing human glutamate receptors. Neuropharmacology. 1997;36:1–11. doi: 10.1016/s0028-3908(96)00160-8. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Santi MR, Neale JH. N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia. 1998;24:172–179. doi: 10.1002/(sici)1098-1136(199810)24:2<172::aid-glia2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wegorzewska IN, Bzdega T, Olszewski RT, Neale JH. Differential negative coupling of type 3 metabotropic glutamate receptor to cyclic GMP levels in neurons and astrocytes. J Neurochem. 2006;96:1071–7. doi: 10.1111/j.1471-4159.2005.03569.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan S, Neale JH. N-Acetylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Saab OH, Neale JH. N-acetylaspartylglutamate inhibits forskolin-stimulated cyclic AMP levels via a metabotropic glutamate receptor in cultured cerebellar granule cells. J Neurochem. 1993;61:943–8. doi: 10.1111/j.1471-4159.1993.tb03606.x. [DOI] [PubMed] [Google Scholar]
- Yao HH, Ding JH, Zhou F, Wang F, Hu LF, Sun T, Hu G. Enhancement of glutamate uptake mediates the neuroprotection exerted by activating group II or III metabotropic glutamate receptors on astrocytes. J Neurochem. 2005;92:948–61. doi: 10.1111/j.1471-4159.2004.02937.x. [DOI] [PubMed] [Google Scholar]