Abstract
Should significant pH heterogeneity exist within cells then the simultaneous calculation of intracellular pH from the distribution of a weak acid will give a value closest to the highest pH in the system, whereas calculation from the distribution of a weak base will give a value closer to the lowest pH. These two values should then differ significantly. Intact rat diaphragms were exposed in vitro to varying bicarbonate concentrations (pure metabolic) and CO2 tensions (pure respiratory), and steady-state cell pH was measured simultaneously either by distribution of the weak acid 5,5-dimethyloxazolidine-2,4-dione-14C (pH DMO) or by distribution of the weak base nicotine-14C (pH nicotine). The latter compound was found suitable to measure cell pH since it was neither metabolized nor bound by rat diaphragms.
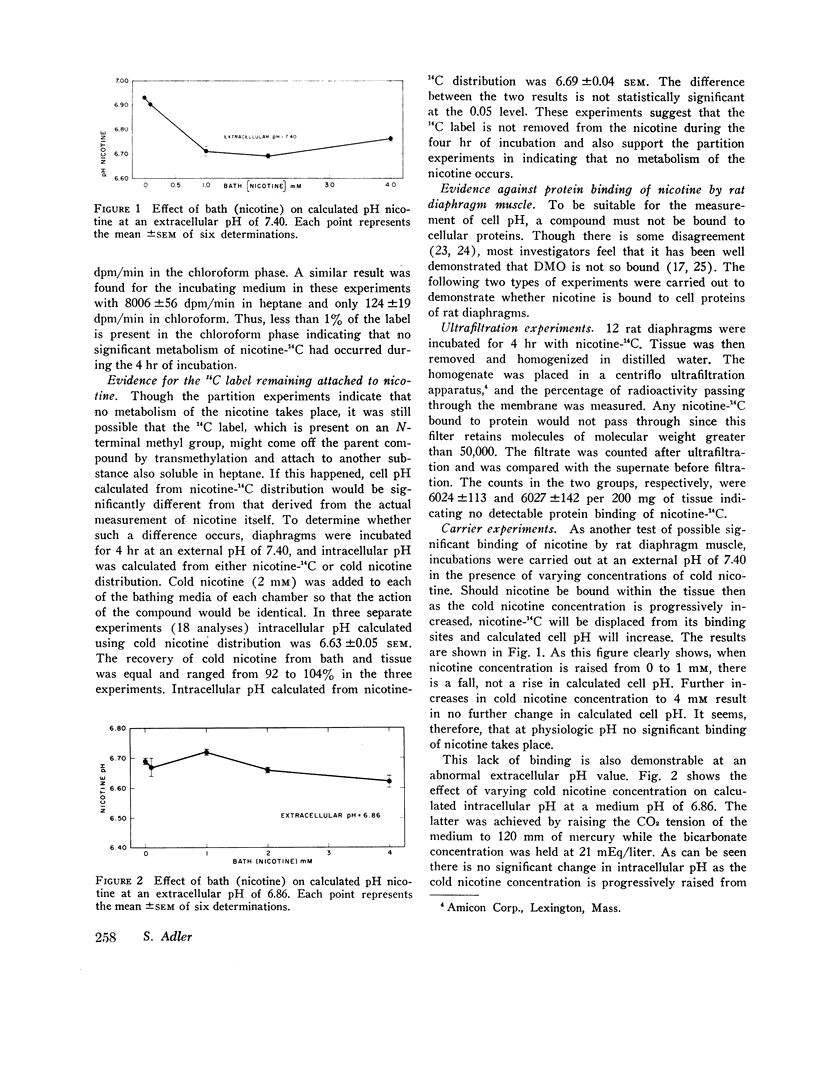
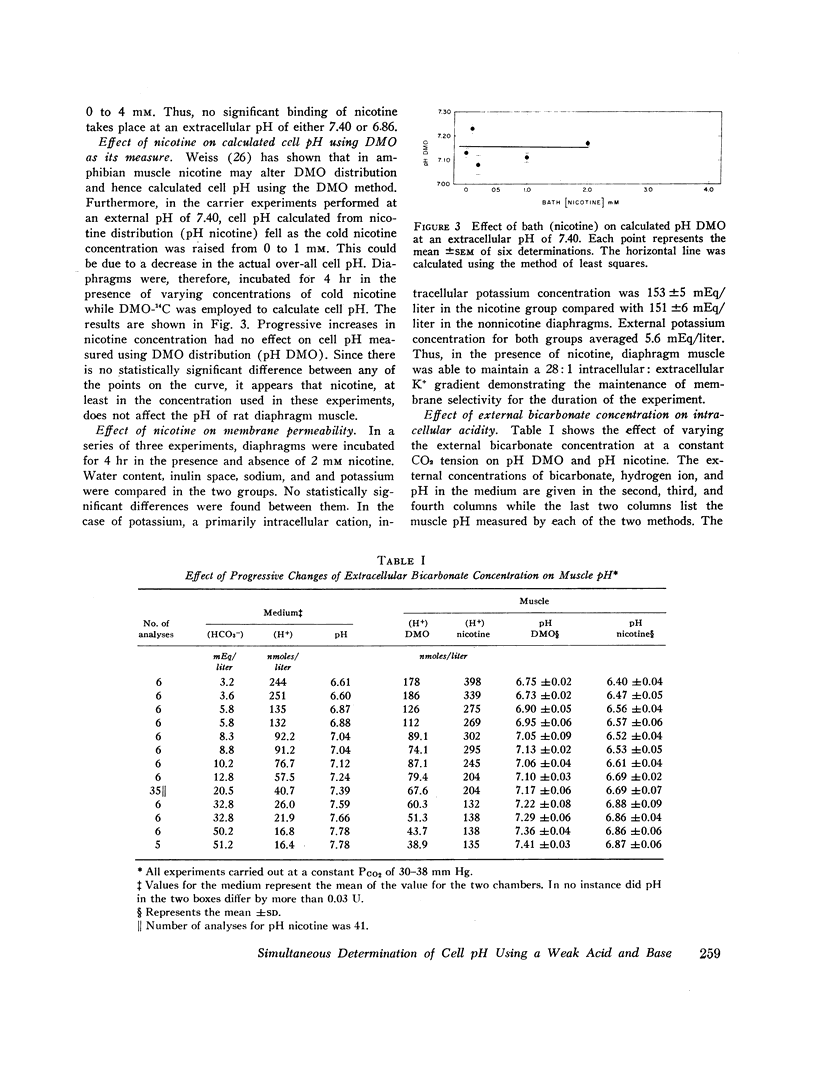
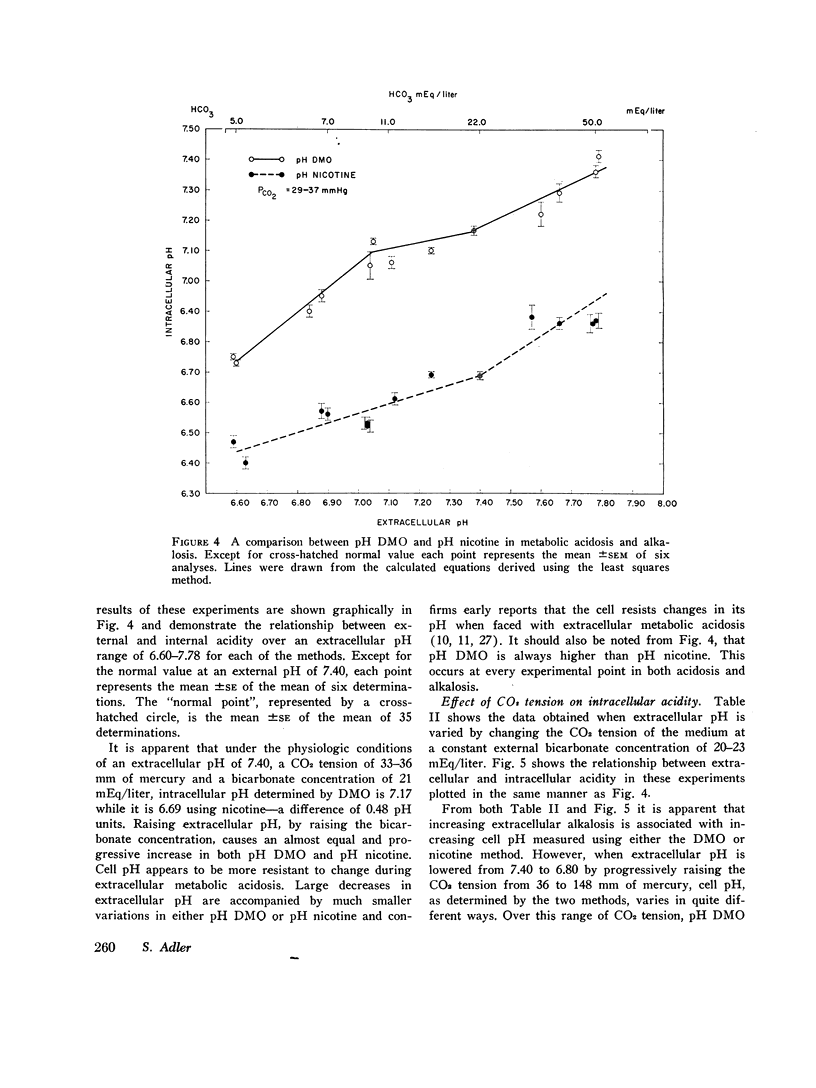
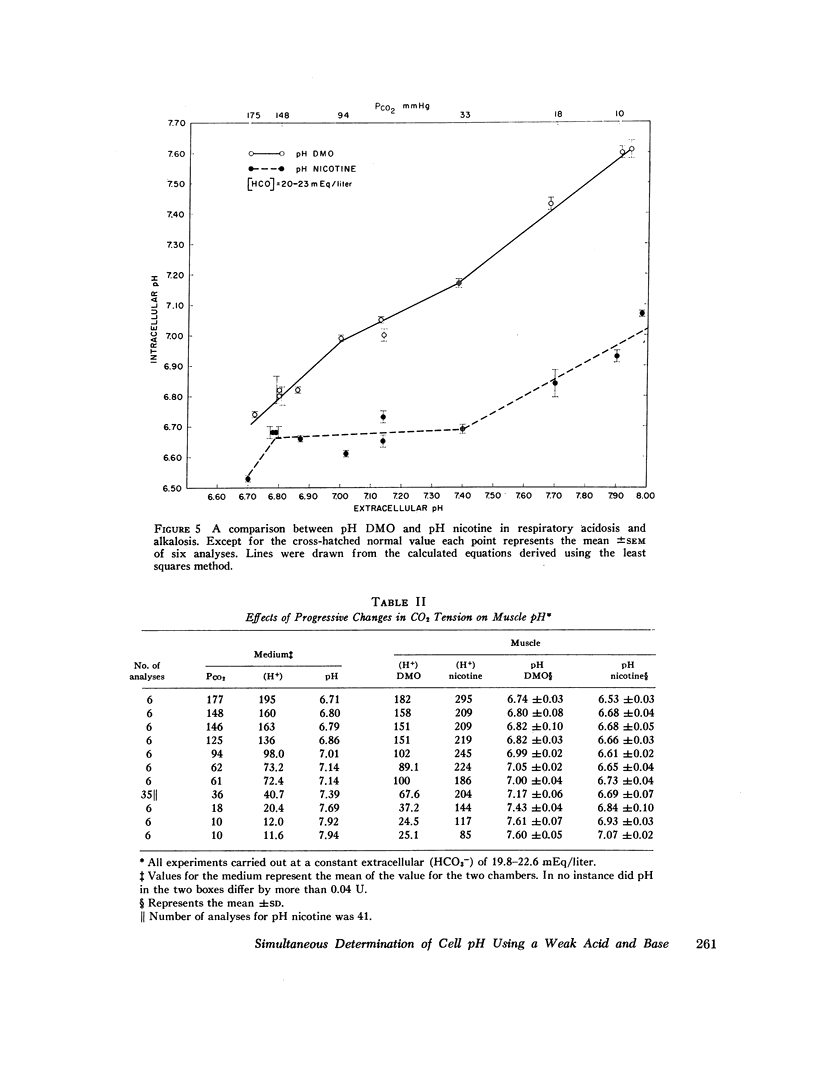
At an external pH of 7.40, pH DMO was 7.17 while pH nicotine was 6.69—a pH difference of 0.48 pH units (P < 0.001). In either respiratory or metabolic alkalosis both DMO and pH nicotine rose so that differences between them remained essentially constant. Metabolic acidosis induced a decrease in both values though they fell more slowly than did extracellular pH. In contradistinction, in respiratory acidosis, decreasing extracellular pH from 7.40 to 6.80 resulted in 0.35 pH unit drop in pH DMO while pH nicotine remained constant. In every experiment, under all external conditions, pH DMO exceeded pH nicotine.
These results indicate that there is significant pH heterogeneity within diaphragm muscle, but the degree of heterogeneity may vary under different external conditions. The metabolic implications of these findings are discussed. In addition, the data show that true overall cell pH is between 6.69 and 7.17—a full pH higher than would be expected from thermodynamic considerations alone. This implies the presence of active processes to maintain cell pH.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. I. THE RESPONSE OF MUSCLE CELLS TO CHANGES IN CO2 TENSION OR EXTRACELLULAR BICARBONATE CONCENTRATION. J Clin Invest. 1965 Jan;44:8–20. doi: 10.1172/JCI105129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ADLER S., ROY A., RELMAN A. S. INTRACELLULAR ACID-BASE REGULATION. II. THE INTERACTION BETWEEN CO-2 TENSION AND EXTRACELLULAR BICARBONATE IN THE DETERMINATION OF MUSCLE CELL PH. J Clin Invest. 1965 Jan;44:21–30. doi: 10.1172/JCI105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addanki S., Cahill F. D., Sotos J. F. Intramitochondrial pH and intra-extramitochondrial pH gradient of beef heart mitochondria in various functional states. Nature. 1967 Apr 22;214(5086):400–402. doi: 10.1038/214400b0. [DOI] [PubMed] [Google Scholar]
- Adler S. An extrarenal action of aldosterone on mammalian skeletal muscle. Am J Physiol. 1970 Mar;218(3):616–621. doi: 10.1152/ajplegacy.1970.218.3.616. [DOI] [PubMed] [Google Scholar]
- Adler S. The role of pH, PCO2, and bicarbonate in regulating rat diaphragm citrate content. J Clin Invest. 1970 Sep;49(9):1647–1655. doi: 10.1172/JCI106382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN E. B., Jr, GOOTT B. Intrcellular hydrogen ion changes and potassium movement. Am J Physiol. 1963 May;204:765–770. doi: 10.1152/ajplegacy.1963.204.5.765. [DOI] [PubMed] [Google Scholar]
- Burnell J. M. In vivo response of muscle to changes in CO2 tension or extracellular bicarbonate. Am J Physiol. 1968 Dec;215(6):1376–1383. doi: 10.1152/ajplegacy.1968.215.6.1376. [DOI] [PubMed] [Google Scholar]
- Butler T. C., Waddell W. J., Poole D. T. Intracellular pH based on the distribution of weak electrolytes. Fed Proc. 1967 Sep;26(5):1327–1332. [PubMed] [Google Scholar]
- Carter N. W., Rector F. C., Jr, Campion D. S., Seldin D. W. Measurement of intracellular pH of skeletal muscle with pH-sensitive glass microelectrodes. J Clin Invest. 1967 Jun;46(6):920–933. doi: 10.1172/JCI105598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B., Mela L. Intramitochondrial pH changes in cation accumulation. Proc Natl Acad Sci U S A. 1966 May;55(5):1243–1251. doi: 10.1073/pnas.55.5.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effros R. M., Chinard F. P. The in vivo pH of the extravascular space of the lung. J Clin Invest. 1969 Nov;48(11):1983–1996. doi: 10.1172/JCI106164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUCKER H. B., GILLETTE J. R., BRODIE B. B. Enzymatic pathway for the formation of cotinine, a major metabolite of nicotine in rabbit liver. J Pharmacol Exp Ther. 1960 May;129:94–100. [PubMed] [Google Scholar]
- KATZMAN R., VILLEE C. A., BEECHER H. K. Effect of increased carbon dioxide concentrations on fixed acid production in vitro. Am J Physiol. 1953 Feb;172(2):317–323. doi: 10.1152/ajplegacy.1953.172.2.317. [DOI] [PubMed] [Google Scholar]
- LONGMORE W. J., HASTINGS A. B., MAHOWALD T. A. EFFECT OF ENVIRONMENTAL CO2 AND PH ON GLYCEROL METABOLISM BY RAT LIVER IN VITRO. J Biol Chem. 1964 Jun;239:1700–1704. [PubMed] [Google Scholar]
- Lennon E. J., Lemann J., Jr Defense of hydrogen ion concentration in chronic metabolic acidosis. A new evaluation of an old approach. Ann Intern Med. 1966 Aug;65(2):265–274. doi: 10.7326/0003-4819-65-2-265. [DOI] [PubMed] [Google Scholar]
- Longmore W. J., Niethe C. M., McDaniel M. L. Effect of CO2 concentration on intracellular pH and on glycogen synthesis from glycerol and glucose in isolated perfused rat liver. J Biol Chem. 1969 Dec 10;244(23):6451–6457. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Novotný I. pH changes during splitting of ATP in skeletal and cardiac muscle extracts and microsomes. Physiol Bohemoslov. 1968;17(6):569–575. [PubMed] [Google Scholar]
- RELMAN A. S., GORHAM G. W., LEVINSKY N. G. The relation between external potassium concentration and the electrolyte content of isolated rat muscle in the steady state. J Clin Invest. 1961 Feb;40:386–393. doi: 10.1172/JCI104265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and intracellular buffering power of the cat brain. Am J Physiol. 1965 Dec;209(6):1233–1246. doi: 10.1152/ajplegacy.1965.209.6.1233. [DOI] [PubMed] [Google Scholar]
- SCHLOERB P. R., GRANTHAM J. J. INTRACELLULAR PH MEASUREMENT WITH TRITIATED WATER, CARBON-14 LABELED 5,5-DIMETHYL-2, 4-OXAZOLIDINEDIONE, AND CHLORIDE-36. J Lab Clin Med. 1965 Apr;65:669–676. [PubMed] [Google Scholar]
- SULLIVAN W. J., DORMAN P. J. The renal response to chronic respiratory acidosis. J Clin Invest. 1955 Feb;34(2):268–276. doi: 10.1172/JCI103080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silman H. I., Karlin A. Effect of local pH changes caused by substrate hydrolysis on the activity of membrane-bound acetylcholinesterase. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1664–1668. doi: 10.1073/pnas.58.4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOBIN R. B. Plasma, extracellular and muscle electrolyte responses to acute metabolic acidosis. Am J Physiol. 1956 Jul;186(1):131–138. doi: 10.1152/ajplegacy.1956.186.1.131. [DOI] [PubMed] [Google Scholar]
- Trivedi B., Danforth W. H. Effect of pH on the kinetics of frog muscle phosphofructokinase. J Biol Chem. 1966 Sep 10;241(17):4110–4112. [PubMed] [Google Scholar]
- Turner D. M. The metabolism of [14C] nicotine in the cat. Biochem J. 1969 Dec;115(5):889–896. doi: 10.1042/bj1150889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UTTER M. F. The role of CO2 fixation in carbohydrate utilization and synthesis. Ann N Y Acad Sci. 1959 Feb 6;72(12):451–461. doi: 10.1111/j.1749-6632.1959.tb44173.x. [DOI] [PubMed] [Google Scholar]
- WADDELL W. J., BUTLER T. C. Calculation of intracellular pH from the distribution of 5,5-dimethyl-2,4-oxazolidinedione (DMO); application to skeletal muscle of the dog. J Clin Invest. 1959 May;38(5):720–729. doi: 10.1172/JCI103852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. J., Bates R. G. Intracellular pH. Physiol Rev. 1969 Apr;49(2):285–329. doi: 10.1152/physrev.1969.49.2.285. [DOI] [PubMed] [Google Scholar]
- Weiss G. B. Dependence of nicotine-C14 distribution and movements upon pH in frog sartorius muscle. J Pharmacol Exp Ther. 1968 Mar;160(1):135–147. [PubMed] [Google Scholar]


