Table 3.
N8-Substituted Pyrimido[5,4-e]-1,2,4-triazine-5,7(6H,8H)-diones 11 from Regiospecific Alkylation of 10
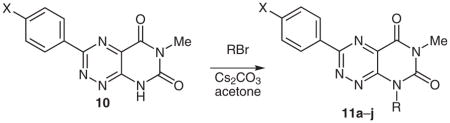 | |||||
|---|---|---|---|---|---|
| Entry | Substrate | X | R | Product | Yield (%) |
| 1 | 10q | Cl | Pr | 11a | 47 |
| 2 | 10s | OMe | (CH2)2NEt2a | 11b | 22 |
| 3 | 10n | H | (CH2)2OH | 11c | 43 |
| 4 | 10n | H | (CH2)2Ph | 11d | 39 |
| 5 | 10p | F | 4-t-BuC6H4CH2 | 11e | 71 |
| 6 | 10q | Cl | 2-FC6H4CH2 | 11f | 57 |
| 7 | 10n | H | 3-FC6H4CH2 | 11g | 66 |
| 8 | 10s | OMe | 4-FC6H4CH2 | 11h | 81 |
| 9 | 10p | F | 3,4-F2C6H3CH2 | 11i | 91 |
| 10 | 10s | OMe | 3,4-F2C6H3CH2 | 11j | 87 |
Chloride reacted instead of bromide.
