Abstract
Background: Soy isoflavones are naturally occurring phytochemicals with weak estrogenic cellular effects. Despite numerous clinical trials of short-term isoflavone supplementation, there is a paucity of data regarding longer-term outcomes and safety.
Objective: Our aim was to evaluate the clinical outcomes of soy hypocotyl isoflavone supplementation in healthy menopausal women as a secondary outcome of a trial on bone health.
Design: A multicenter, randomized, double-blind, placebo-controlled 24-mo trial was conducted to assess the effects of daily supplementation with 80 or 120 mg aglycone equivalent soy hypocotyl isoflavones plus calcium and vitamin D on the health of 403 postmenopausal women. At baseline and after 1 and 2 y, clinical blood chemistry values were measured and a well-woman examination was conducted, which included a mammogram and a Papanicolaou test. A cohort also underwent transvaginal ultrasound measurements to assess endometrial thickness and fibroids.
Results: The baseline characteristics of the groups were similar. After 2 y of daily isoflavone exposure, all clinical chemistry values remained within the normal range. The only variable that changed significantly was blood urea nitrogen, which increased significantly after 2 y (P = 0.048) but not after 1 y (P = 0.343) in the supplementation groups. Isoflavone supplementation did not affect blood lymphocyte or serum free thyroxine concentrations. No significant differences in endometrial thickness or fibroids were observed between the groups. Two serious adverse events were detected (one case of breast cancer and one case of estrogen receptor–negative endometrial cancer), which was less than the expected population rate for these cancers.
Conclusion: Daily supplementation for 2 y with 80–120 mg soy hypocotyl isoflavones has minimal risk in healthy menopausal women. This trial was registered at clinicaltrials.gov as NCT00665860
INTRODUCTION
Soy (Glycine max) contains isoflavones, which are naturally occurring plant estrogens with chemical structures similar to the mammalian estrogens secreted from the ovaries. After menopause, the time when a woman's ovaries no longer produce estrogen, some women experience severe symptoms related to hot flashes, genital atrophy, and osteoporosis. Traditional hormone therapy (HT) with mammalian estrogens is very effective in treating menopausal symptoms, but it is associated with an increased risk of breast cancer and, when unopposed with a progestrogen, endometrial cancer (1–3). In addition, the previously presumed benefit of HT on the risk of cardiovascular disease was not confirmed in the Women's Health Initiative (2). Soy food products and isoflavone supplements have been proposed as alternatives to HT.
Very few reports of serious adverse health effects from isoflavone supplementation have been reported in the medical literature or from clinical trials. However, as the commercial popularity of soy isoflavone supplements continues to grow, so does the concern regarding whether chronic exposure is safe at levels exceeding those obtained from the diet (4–8). Unlike food products derived from soy, such as tofu, soy isoflavone tablets allow for the potential of ingesting large amounts of isoflavones per day. For instance, soy isoflavone tablets allow menopausal women to consume 50–120 mg isoflavone/d, which is 1–4 times as much isoflavone exposure from typical Asian diets and up to 100 times more exposure than from a typical Western diet (9, 10). Although case reports exist of adverse effects when menopausal women chronically ingest large amounts of isoflavones (not specifically soy isoflavones) (11), a recent 3-mo trial of menopausal women taking high daily doses of soy isoflavones (900 mg) revealed only few and mild adverse effects (12). Although numerous studies, including significant randomized controlled trials (RCTs), have examined the potential health benefits of soy isoflavone supplementation for menopausal women, very few have specifically targeted clinical outcomes and safety assessments over a relatively longer period of time (13–22).
We conducted one of the largest and most comprehensive RCTs to date in which we examined the risks and benefits of soy intake in menopausal women, known as the Osteoporosis Prevention Using Soy (OPUS) Study (23). The OPUS Study compared placebo with daily supplementation of soy hypocotyl isoflavones (the equivalent of 80 or 120 mg aglycones), administered primarily as their β-glucoside conjugates in healthy menopausal women over a 2-y interval. The primary outcome measure revealed that 120 mg soy isoflavones reduce whole body bone loss but do not slow bone loss at common fracture sites. The major secondary outcome measured in the OPUS Study was safety. An investigation of mammographic density, a marker of breast cancer risk, found that 2 y of supplementation did not adversely modify breast density in this population of postmenopausal women (24). This manuscript examined clinical blood chemistry measures, well-woman examination results, and adverse events to provide an assessment of clinical outcomes of soy hypocotyl isoflavone supplementation in a population of healthy menopausal women.
SUBJECTS AND METHODS
Study design
The OPUS study was a multicenter, randomized, double-blind, placebo-controlled, 2-y intervention trial conducted between March 2002 and June 2006 at Baylor College of Medicine (study coordinating center), at the University of California at Davis in collaboration with the Kaiser Foundation Research Institute, and at the University of Georgia. Study subjects provided written informed consent before any study activities were conducted, and the study's protocol was approved by the institutional review board for human studies at each institution.
The study design was previously described (23), but is included in part here for clarity. Menopausal women between the ages of 40 and 60 y with a serum follicle-stimulating hormone concentration of >30 IU/mL and ≥12 mo of amenorrhea were eligible to participate. Women who had undergone a bilateral oophorectomy ≥6 mo previously also qualified if the other study criteria were met. Eligible women included nonrunners and nonvegetarians, and soy food consumption of less than one serving per week was acceptable. Exclusion criteria included an abnormal result from a screening mammogram, a Papanicolau test, or blood chemistry test; a body mass index (BMI; in kg/m2) >30; smoking; a history of osteoporosis, spine, and/or hip fracture or cancer; and active liver, kidney, gallbladder, or heart disease. Osteopenic women (lumbar spine bone mineral density T score < −1.5) were excluded from the study. Also excluded were women being treated with medications that alter bone metabolism, such as bisphosphonates, calcitonin, fluoride, corticosteroids, tamoxifen, raloxifene, letrozole, or HT. If they agreed to stop the medication or treatment for 3 mo, they would be considered for participation in the study. At the end of the 3-mo washout period, they must still have met the other study inclusion criteria, agreed to withhold the medication or treatment during the study period, and provided written permission from their primary care physicians. Women taking herbal supplements known to alter bone metabolism or to have estrogenic effects, such as black cohosh, blue cohosh, dong quai, Caltrate 600+Soy (Wyeth Consumer Health Care, Richmond, VA), Estroven (Amerifit Brands, Cromwell, CT), ginseng, Healthy Women (Johnson & Johnson, Langhome, PA), Opti-Soy (Optimum Nutrition Inc, Aurora, IL), PhytoFem (The Nutri Centre, London, United Kingdom), Probalance (AntiAging Systems, Sark, United Kingdom), Promensil (Natrol Inc, Chatsworth, CA), Remifemin (Enzymatic Therapy Inc, Green Bay, WI), Rimostil (Novogen Inc, New Canaan, CT), or Trinovin (Novogen Inc) were excluded. Women taking these supplements would be considered for participation if they agreed to stop taking the supplements for 6 wk and continue to do so during the study period and if they met all other study inclusion criteria.
The study consisted of 2 periods: a screening phase and a double-blind study period of 24 mo. Informed consent was obtained, after which the subjects received a baseline screening mammogram, a physical examination that included a Papanicolau test, a stool guaiac test, clinical blood chemistry measures, and dual-energy X-ray absorptiometry scans. After screening, those women who met the study criteria entered the study. Approximately 135 women at each study site were randomly assigned within 9 of 15 time blocks so that one-third of the participants were assigned to receive 80 mg soy hypocotyl isoflavones/d, one-third to receive 120 mg soy hypocotyl isoflavones/d, and the remaining one-third to receive a placebo. All investigators, research staff, and subjects were blinded to the treatment codes. Procedures to limit variability in a long-term trial were used, such as analyzing research samples for all time points for a given subject at one time.
Study visits took place at baseline and at 1 and 2 y. Dual-energy X-ray absorptiometry measurements, blood drawings, inquiries about supplement use, and well-women examinations were carried out at these time points at each research site, as previously described (23). Metrics for safety were adopted from published guidelines of annual screening in menopausal women and based on questions raised from prior soy studies (25–27). Additionally, subjects at the California site were invited to participate in clinical examinations at each study visit to evaluate the effects of the isoflavone supplements on endometrial thickness and uterine fibroids, if present. Each woman completed a soy food questionnaire at baseline, which provided information regarding usual soy consumption, which was adjusted for in the final statistical analysis. The questionnaire was analyzed by the Fred Hutchinson Cancer Research Center Nutrition Assessment Shared Resource (Seattle, WA). The staff at the Nutrition Assessment Shared Resource was not involved in the study and was blinded to the treatment codes.
Soy isoflavone and placebo tablets
The soy hypocotyl isoflavones were manufactured and provided by Frutarom Netherlands BV (Veenendaal, Netherlands), whereas the placebo and isoflavone-containing tablets were manufactured and packaged by Pharma Consulting & Industries BV (Eede, Netherlands). The isoflavone aglycone and glucoside contents were measured by Nutrilab BV (Giessen, Netherlands) by using an HPLC method based on Song et al (28). Two batches (year 1 and year 2 tablets) were used to support the study. The isoflavone content of the year 2 tablets was slightly higher, with the average content of each isoflavone tablet containing 40.51 mg aglycone equivalent of total isoflavones (daidzein: 22.01 mg; glycitein: 13.54 mg; genistein: 4.96 mg) with the majority (>95%) in the form of glycosides reflecting the natural composition of the soy germ. Year 1 tablets, by comparison, had an average content of 34.10 mg aglycone equivalent of total isoflavones (daidzein: 17.87 mg; glycitein: 11.22 mg; genistein: 5.00 mg). The difference between the batches reflects inherent differences in the crop of soybeans used for processing. The placebo tablets contained <1.0 mg aglycone equivalent per tablet. The placebo and isoflavone tablets each contained the same filler materials and common processing aids, yielding tablets that were identical in appearance. Each woman ingested 3 tablets each day, one with each main meal.
Compliance with the study supplement was assessed by pill counts of empty pill blister packs and remaining pills at 6, 12, 18, and 24 mo and calculated by using the following formula: (number of tablets dispensed – number of tablets returned/number of tablets subject was expected to take) × 100. Compliance was further validated by measuring blood isoflavone concentrations at baseline and at each annual visit. Serum isoflavones were measured by HPLC-mass spectrometry as previously described (29).
Additionally, each woman in the treatment and placebo groups was supplemented daily with 1000 mg calcium carbonate (400 mg Ca) provided by Source Natural (Scotts Valley, CA) and a one-a-day multivitamin provided by Swanson Health Products (Fargo, ND) that delivered 400 IU vitamin D. This was done to minimize individual dietary intake differences and to ensure an intake that approximated the Dietary Reference Intake guidelines for the study population. The design sought to standardize intakes of calcium and vitamin D, nutrients known to have an effect on bone metabolism, and to minimize its potential effect on the primary study outcome, bone mineral density. The composition of the multivitamin and mineral supplement (Swanson Premium Century Formula Without Iron): vitamin A USP (as acetate) 5000 IU, vitamin C USP (as ascorbic acid) 90 mg, vitamin D USP (as cholecalciferol) 400 IU, vitamin E USP (as d-α-tocopheryl acetate) 30 IU, vitamin K (as phytonadione) 25 μg, thiamine USP (thiamine mononitrate, vitamin B-1) 2.25 mg, riboflavin USP (vitamin B-2) 2.6 mg, niacinamide USP 20 mg, vitamin B-6 USP (as pyridoxine HCl) 3 mg, folic acid USP 400 μg, vitamin B-12 USP (as cyanocobalamin) 9 μg, Biotin USP 25 μg, pantothenic acid USP (as d-calcium pantothenate) 10 mg, calcium (from dicalcium phosphate) 162 mg, iodine USP (from potassium iodide) 150 μg, magnesium (from magnesium oxide) 100 mg, zinc (from zinc sulfate) 15 mg, selenium (from sodium selenate) 25 μg, copper (from copper gluconate) 2 mg, manganese (from manganese sulfate) 5 mg, chromium (from chromium chloride) 25 μg, molybdenum (from sodium molybdate) 25 μg, and potassium (from potassium chloride) 57.2 mg.
Clinical blood chemistry and examination analyses
Serum samples were analyzed for complete blood count, serum electrolytes, thyroid function, gonadotropins (follicle-stimulating hormone and luteinizing hormone), estradiol, liver function, and renal and lipid profiles. Certified clinical laboratories at each study site performed the measurements according to clinically accepted standardized analytic protocols.
Transvaginal ultrasonographic measurement of the midsagittal endometrial thickness was performed by one physician blinded to treatment groups on subjects in the California cohort to assess the effects of soy hypocotyl isoflavone supplementation on endometrial thickness and the growth of uterine fibroids, if present.
Clinical outcomes and safety monitoring
Clinical outcomes such as clinical chemistry profiles, annual well-woman examination results, and mammogram results and adverse events were monitored throughout the study by clinical coordinators, study physicians, and principal investigators. Each physician used a standardized medical history and physical examination form and protocol adopted for the study. Information gathered during the annual examination included assessment of constitutional findings and organ systems (head and neck, thyroid, breast, respiratory system, cardiovascular system, abdomen, extremities, skin, central nervous system, musculoskeletal, gastrointestinal, and urogenital system). Team physicians communicated any clinically significant findings to the study participants and recommended follow-up with their personal physicians. Participant's self-reported adverse health events and concerns were evaluated by investigators to determine their seriousness and relation to the study protocol. Serious adverse events were reported to our independent Data Safety Monitoring Board, funding agency, and respective institutional review board committees.
Statistical analysis
Treatment groups were compared with respect to baseline demographic and clinical characteristics for potential confounding. Those characteristics shown to be different between groups to a clinically important degree were included as covariates in the analyses. Each outcome was assessed by using generalized estimating equations with respect to the effects of treatment group, time, and interaction between treatment and time with clinical site, confounders, and the corresponding baseline value of the outcome variable accounted for (30). For measures that were performed only at the University of California, Davis, and Kaiser Foundation Research Institute center, study site was not included in the statistical model. Log transformations of skewed data were carried out as needed, and the statistical analyses were repeated. A significant treatment-by-time interaction was followed by group comparison at years 1 and 2. If the interaction was not significant, that term was dropped from the model and the analysis was repeated to assess the main effect of treatment. Bonferroni adjustments were made for testing multiple comparisons. Analyses were performed with SPSS software (version 16: SPSS Inc, Chicago, IL) and the sequential Sidak multiple comparison procedure was applied to all pairwise comparisons between the 3 treatment groups.
The manufacturers of the supplements were not involved in the study design or data analysis. The academic authors had full and unrestricted rights to analyze, interpret, and publish the results. All key investigators remained blinded throughout the statistical analyses.
RESULTS
Study subjects
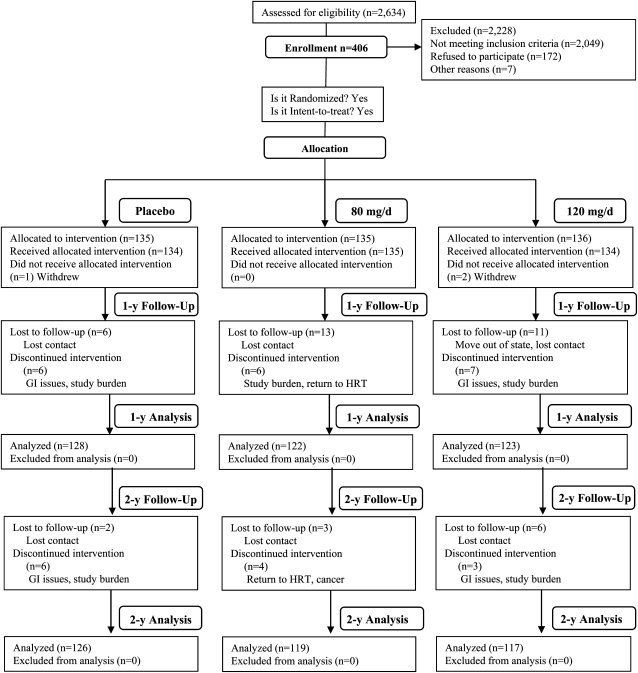
A total of 406 women were enrolled and randomly assigned to treatment groups; 403 subjects actually received the allocated intervention; 134 women in the placebo, 135 in the 80-mg soy hypocotyl isoflavone/d, and 134 in the 120-mg soy hypocotyl isoflavone/d treatment groups. As shown in Figure 1, the attrition rates at both years 1 and 2 were low and were not significantly different between the treatment groups. Common reasons for withdrawal from the study included study burden, gastrointestinal upset and desire to return to HT. The baseline characteristics of the study subjects are provided in Table 1. Age, age at menarche, age at menopause, height, weight, BMI, body composition, body temperature, pulse rate, and blood pressure were similar at baseline between the treatment groups.
FIGURE 1.
Flow chart of the study design and subject participation throughout the study. GI, gastrointestinal; HRT, hormone replacement therapy. Reproduced with permission from reference 23.
TABLE 1.
Baseline characteristics of study subjects
| Soy isoflavone–treated groups | ||||
| Placebo group | 80 mg/d | 120 mg/d | P value1 | |
| n | 134 | 135 | 134 | |
| Age (y) | 55.0 ± 3.72 | 54.9 ± 4.0 | 54.5 ± 4.1 | 0.55 |
| Age at menarche (y) | 12.7 ± 1.5 | 12.9 ± 1.5 | 12.8 ± 1.6 | 0.46 |
| Age at menopause (y) | 48.2 ± 5.1 | 48.5 ± 5.5 | 47.6 ± 6.3 | 0.42 |
| Weight (kg) | 68.0 ± 9.8 | 68.8 ± 13.2 | 67.9 ± 10.2 | 0.766 |
| Height (cm) | 163.8 ± 6.7 | 164.8 ± 5.9 | 165.0 ± 6.0 | 0.236 |
| BMI (kg/m2) | 25.4 ± 3.4 | 25.3 ± 4.5 | 24.9 ± 3.2 | 0.594 |
| Lean body mass (kg) | 41.4 ± 4.3 | 42.4 ± 4.8 | 42.2 ± 4.8 | 0.166 |
| Body fat (kg) | 24.9 ± 6.0 | 25.0 ± 6.4 | 24.4 ± 6.9 | 0.734 |
| Body fat (%) | 36.5 ± 5.3 | 35.6 ± 5.4 | 35.7 ± 5.9 | 0.406 |
| Body temperature (°F) | 97.6 ± 0.9 | 97.6 ± 0.8 | 97.6 ± 1.4 | 0.63 |
| Pulse (beats/min) | 72.5 ± 12.2 | 73.2 ± 10.7 | 73.3 ± 9.3 | 0.81 |
| Blood pressure (mm Hg) | ||||
| Systolic | 122.9 ± 16.2 | 122.4 ± 16.3 | 119.7 ± 15.8 | 0.23 |
| Diastolic | 71.0 ± 10.3 | 71.5 ± 10.3 | 70.6 ± 11.6 | 0.81 |
P values were derived by one-factor ANOVA between the 3 treatment groups.
Mean ± SD (all such values).
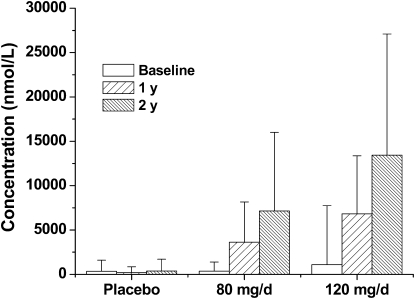
Subject compliance with the supplement protocol was 95% at year 1 and 99% at year 2, which was based on self-reports and pill counts of returned packages and unused pills every 6 mo. Compliance with the intervention by study participants was confirmed by blood isoflavone concentrations. As shown in Figure 2, blood concentrations of daidzein at both year 1 and 2 were dramatically (P < 0.001) higher than those at baseline and those in the placebo group at all time points, which indicated a significant (P < 0.001) dose-dependent response. The elevated blood concentrations of daidzein after 2 y of treatment could be accounted for by the higher daidzein content in the second batch of soy isoflavone tablets (23% greater than in year 1) and by the better compliance among study participants who remained on the project. Genistein and glycetin exhibited similar response pattern (data not shown). The equol-producing capabilities of the subjects were assessed at baseline and after years 1 and 2. The blood concentrations of equol were similar to the pattern reported for daidzein, because daidzein is a precursor for equol. Approximately 33% of the study subjects were equol producers. This percentage is consistent with that (20–35%) reported among Western adult populations (31).
FIGURE 2.
Mean (±SD) fasting blood concentrations of daidzein in participants at baseline and after 1 and 2 y of treatment. Blood concentrations of daidzein were compared between the 3 treatment groups over time by generalized estimating equation (GEE) techniques to assess the effect of group, time, and group × time interaction with repeated measures accounted for. A significant group × time interaction (P < 0.001) was detected; therefore, groups were compared at each time point by using contrasts and multiple comparison procedures provided by GEE analysis. The data were log transformed before analysis because of a highly skewed distribution. The blood concentrations of daidzein after 1 and 2 y of isoflavone treatment (80 or 120 mg/d) were significantly (P < 0.001) greater than their respective baseline values. The blood concentrations of daidzein in subjects receiving the 120-mg/d treatment were also significantly greater (P < 0.001) than the respective values in subjects who received the 80-mg/d treatment.
Clinical blood chemistry values
The clinical laboratory values indicated minimal changes in response to soy hypocotyl isoflavone supplementation (Tables 2 and 3). The interactions with group across time were not significant, and the multivariate model P values for years 1 and 2 after adjustment for covariates were consistently not significant. No clinically or statistically significant changes in the complete blood count, serum electrolytes, liver function test results, or lipid panel results. The subject population consisted of healthy menopausal women, which was further supported by the lipid results, particularly HDL cholesterol. Serum free thyroxine (FT4) concentrations were slightly lower and nearly statistically significantly different between the treatment groups and the placebo group after 2 y (P = 0.052), but not after 1 y (P = 0.739), and the changes were not dose-dependent. Thyroid-stimulating hormone concentrations did not change significantly. The only variable that changed significantly was blood urea nitrogen (BUN), which increased significantly after 2 y (P = 0.048) but not after 1 y (P = 0.343) in the supplementation groups. Serum creatinine and the BUN:creatinine ratios did not change significantly. Importantly, the concentrations of FT4 and BUN in all 3 groups at all time points remained within the normal laboratory reference ranges.
TABLE 2.
Descriptive statistics for blood chemistry measures for the 3 treatment groups at baseline and after 1 and 2 y of soy isoflavone supplementation1
| Soy isoflavone–treated groups |
||||
| Variables (normal range) and sequence | Placebo group | 80 mg/d | 120 mg/d | 2 |
| CBC | ||||
| WBC (4.5–11.0 × 103/μL) | ||||
| Baseline | 5.4 ± 1.3 | 5.3 ± 1.2 | 5.2 ± 1.3 | 0.544 |
| 1 y | 5.4 ± 1.3 | 5.1 ± 1.2 | 5.1 ± 1.1 | 0.223 |
| 2 y | 5.4 ± 1.3 | 5.2 ± 1.4 | 5.2 ± 1.2 | 0.901 |
| RBC (4.5–5.2 × 106/μL) | ||||
| Baseline | 4.5 ± 0.3 | 4.4 ± 0.3 | 4.5 ± 0.3 | 0.413 |
| 1 y | 4.4 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.3 | 0.161 |
| 2 y | 4.5 ± 0.3 | 4.4 ± 0.3 | 4.4 ± 0.3 | 0.143 |
| Hemoglobin (12.0–16.0 g/dL) | ||||
| Baseline | 13.5 ± 0.8 | 13.5 ± 0 | 13.7 ± 0.9 | 0.357 |
| 1 y | 13.6 ± 0.8 | 13.6 ± 0.9 | 13.6 ± 0.8 | 0.533 |
| 2 y | 13.6 ± 0.9 | 13.6 ± 0.8 | 13.6 ± 0.9 | 0.235 |
| Hematocrit (36–46%) | ||||
| Baseline | 39.8 ± 2.3 | 39.9 ± 2.3 | 40.3 ± 2.5 | 0.290 |
| 1 y | 40.0 ± 2.30 | 40.0 ± 2.5 | 40.1 ± 2.2 | 0.337 |
| 2 y | 39.9 ± 2.5 | 39.9 ± 2.2 | 39.9 ± 2.5 | 0.661 |
| MCV (80–100 fL) | ||||
| Baseline | 89.1 ± 5.1 | 90.2 ± 4.0 | 90.2 ± 4.2 | 0.053 |
| 1 y | 90.0 ± 4.6 | 90.6 ± 6.1 | 91.2 ± 3.7 | 0.575 |
| 2 y | 89.6 ± 4.7 | 90.4 ± 4.1 | 90.7 ± 4.2 | 0.127 |
| MCH (27–33 pg) | ||||
| Baseline | 30.3 ± 1.8 | 30.6 ± 1.4 | 30.6 ± 1.6 | 0.240 |
| 1 y | 30.5 ± 1.8 | 30.9 ± 1.6 | 30.9 ± 1.4 | 0.323 |
| 2 y | 30.5 ± 1.8 | 30.9 ± 1.5 | 30.9 ± 1.6 | 0.514 |
| MCHC (32–36 g/dL) | ||||
| Baseline | 34.0 ± 0.9 | 33.9 ± 0.8 | 34.0 ± 0.8 | 0.613 |
| 1 y | 33.9 ± 0.7 | 33.9 ± 0.7 | 33.9 ± 0.7 | 0.908 |
| 2 y | 34.0 ± 0.8 | 34.1 ± 0.8 | 34.1 ± 0.8 | 0.764 |
| RDW (0–14.7%) | ||||
| Baseline | 13.2 ± 0.9 | 13.3 ± 0.8 | 12.2 ± 0.7 | 0.661 |
| 1 y | 13.3 ± 0.9 | 13.3 ± 0.7 | 13.2 ± 0.8 | 0.597 |
| 2 y | 13.4 ± 0 | 13.4 ± 0.8 | 13.4 ± 0.7 | 0.757 |
| SEG (40–70%) | ||||
| Baseline | 56.2 ± 9.0 | 56.2 ± 9.6 | 55.6 ± 10.3 | 0.827 |
| 1 y | 56.2 ± 9.3 | 56.2 ± 8.0 | 55.7 ± 85 | 0.694 |
| 2 y | 56.0 ± 8.7 | 54.9 ± 9.0 | 55.7 ± 8.6 | 0.467 |
| Lymphocytes (22.2–43.6%) | ||||
| Baseline | 33.7 ± 7.7 | 33.8 ± 8.7 | 34.5 ± 8.9 | 0.710 |
| 1 y | 33.6 ± 8.2 | 33.7 ± 7.6 | 33.8 ± 7.7 | 0.903 |
| 2 y | 33.4 ± 8.2 | 34.4 ± 8.7 | 33.6 ± 8.2 | 0.534 |
| Monocytes (3–12%) | ||||
| Baseline | 6.5 ± 2.4 | 6.5 ± 2.4 | 6.7 ± 2.5 | 0.841 |
| 1 y | 6.7 ± 2.0 | 6.6 ± 2.2 | 6.9 ± 1.9 | 0.521 |
| 2 y | 7.0 ± 2.0 | 7.2 ± 2.1 | 7.2 ± 1.9 | 0.829 |
| Eosinophils (0–7%) | ||||
| Baseline | 3.0 ± 1.8 | 2.8 ± 2.0 | 2.7 ± 1.6 | 0.557 |
| 1 y | 3.1 ± 2.0 | 2.9 ± 1.6 | 3.0 ± 1.9 | 0.521 |
| 2 y | 3.0 ± 1.6 | 2.9 ± 1.7 | 2.9 ± 1.7 | 0.880 |
| Basophils (0–2%) | ||||
| Baseline | 0.8 ± 1.6 | 0.63 ± 0.3 | 0.63 ± 0.4 | 0.375 |
| 1 y | 0.6 ± 0.5 | 0.65 ± 0.5 | 0.59 ± 0.4 | 0.887 |
| 2 y | 0.6 ± 0.4 | 0.6 ± 0.3 | 0.6 ± 0.4 | 0.679 |
| ANC (>1.5 × 103/μL) | ||||
| Baseline | 3.1 ± 1.0 | 3.0 ± 1.1 | 3.0 ± 1.0 | 0.536 |
| 1 y | 3.1 ± 1.1 | 2.9 ± 0.9 | 2.9 ± 0.9 | 0.294 |
| 2 y | 3.1 ± 1.0 | 2.9 ± 1.0 | 2.9 ± 0.9 | 0.547 |
| Platelets (130–400 × 103/μL) | ||||
| Baseline | 274.3 ± 51.2 | 261.3 ± 56.6 | 252.4 ± 49.9 | 0.003 |
| 1 y | 272.1 ± 54.1 | 260.1 ± 63.9 | 248.1 ± 49.8 | 0.334 |
| 2 y | 272.6 ± 53.3 | 259.1 ± 49.2 | 249.9 ± 54.1 | 0.448 |
| MPV (7.5–11.5 fL) | ||||
| Baseline | 8.1 ± 1.0 | 8.2 ± 1.2 | 8.2 ± 1.3 | 0.948 |
| 1 y | 7.9 ± 0.6 | 7.8 ± 0.9 | 7.9 ± 0.7 | 0.810 |
| 2 y | 7.8 ± 0.9 | 8.0 ± 1.2 | 7.8 ± 1.5 | 0.770 |
| Blood chemistry profile | ||||
| Sodium (135–145 mmol/L) | ||||
| Baseline | 140.7 ± 2.2 | 140.9 ± 2.2 | 140.9 ± 2.4 | 0.867 |
| 1 y | 140.4 ± 2.8 | 140.4 ± 1.9 | 140.0 ± 2.8 | 0.224 |
| 2 y | 139.2 ± 11.5 | 140.3 ± 2.2 | 140.2 ± 2.4 | 0.443 |
| Potassium (3.3–5.0 mmol/L) | ||||
| Baseline | 4.4 ± 0.5 | 4.4 ± 0.4 | 4.4 ± 0.4 | 0.854 |
| 1 y | 4.3 ± 0.4 | 4.2 ± 0.4 | 4.3 ± 0.4 | 0.543 |
| 2 y | 4.3 ± 0.4 | 4.3 ± 0.4 | 4.2 ± 0.3 | 0.181 |
| Chlorine (95–110 mmol/L) | ||||
| Baseline | 103.5 ± 2.7 | 103.6 ± 2.7 | 103.5 ± 2.7 | 0.882 |
| 1 y | 103.2 ± 2.8 | 103.6 ± 2.2 | 103.0 ± 2.6 | 0.146 |
| 2 y | 103.6 ± 2.5 | 103.8 ± 2.1 | 103.7 ± 2.5 | 0.881 |
| Calcium (8.6–10.5 mg/dL) | ||||
| Baseline | 9.5 ± 0.3 | 9.5 ± 0.37 | 9.5 ± 0.4 | 0.860 |
| 1 y | 9.6 ± 0.3 | 9.6 ± 0.36 | 9.5 ± 0.4 | 0.201 |
| 2 y | 9.4 ± 0.3 | 9.4 ± 0.36 | 9.4 ± 0.4 | 0.903 |
| Carbon dioxide (24–32 mmol/L) | ||||
| Baseline | 26.4 ± 3.3 | 26.5 ± 3.5 | 26.6 ± 3.3 | 0.856 |
| 1 y | 25.8 ± 3.7 | 25.4 ± 3.8 | 25.5 ± 3.8 | 0.340 |
| 2 y | 25.1 ± 3.6 | 25.4 ± 3.8 | 25.6 ± 3.7 | 0.359 |
| Glucose (70–110 mg/dL) | ||||
| Baseline | 85.8 ± 8.6 | 86.5 ± 8.8 | 84.6 ± 11.5 | 0.241 |
| 1 y | 86.4 ± 12.0 | 88.9 ± 12.9 | 87.0 ± 11.2 | 0.316 |
| 2 y | 88.6 ± 8.7 | 89.7 ± 13.2 | 88.4 ± 11.8 | 0.964 |
| Renal function | ||||
| BUN (8–22 mg/dL) | ||||
| Baseline | 14.3 ± 3.7 | 13.8 ± 3.6 | 14.3 ± 3.9 | 0.446 |
| 1 y | 14.4 ± 4.1 | 14.1 ± 3.6 | 14.9 ± 3.3 | 0.343 |
| 2 y | 14.4 ± 3.9 | 14.5 ± 3.8 | 15.4 ± 3.4 | 0.048 |
| Creatinine (0.5–1.3 mg/dL) | ||||
| Baseline | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.281 |
| 1 y | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.596 |
| 2 y | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.145 |
| BUN:creatinine | ||||
| Baseline | 18.0 ± 5.3 | 16.8 ± 4.9 | 17.4 ± 4.6 | 0.180 |
| 1 y | 17.5 ± 5.5 | 16.8 ± 4.2 | 18.0 ± 4.4 | 0.411 |
| 2 y | 17.6 ± 5.1 | 17.0 ± 4.0 | 18.0 ± 4.0 | 0.504 |
| Liver function | ||||
| Globulin (2.3–3.5 g/L) | ||||
| Baseline | 2.7 ± 0.4 | 2.7 ± 0.4 | 2.7 ± 0.3 | 0.458 |
| 1 y | 2.7 ± 0.3 | 2.6 ± 0.3 | 2.7 ± 0.3 | 0.157 |
| 2 y | 2.8 ± 0.3 | 2.8 ± 0.3 | 2.8 ± 0.3 | 0.975 |
| Albuminoglobulin (3.5–5.0 g/L) | ||||
| Baseline | 1.6 ± 0.4 | 1.6 ± 0.2 | 1.6 ± 0.3 | 0.790 |
| 1 y | 1.6 ± 0.2 | 1.7 ± 0.3 | 1.6 ± 0.4 | 0.166 |
| 2 y | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.5 ± 0.2 | 0.870 |
| Bilirubin, total (0.3–1.3 mg/dL) | ||||
| Baseline | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.590 |
| 1 y | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.5 | 0.969 |
| 2 y | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.6 ± 0.3 | 0.235 |
| ALT (5–54 U/L) | ||||
| Baseline | 25.6 ± 11.7 | 25.0 ± 11.9 | 23.4 ± 15.0 | 0.385 |
| 1 y | 23.5 ± 9.4 | 22.0 ± 6.7 | 22.9 ± 10.5 | 0.412 |
| 2 y | 22.5 ± 8.6 | 23.2 ± 16.1 | 21.0 ± 7.5 | 0.396 |
| AST (15–43 U/L) | ||||
| Baseline | 23.7 ± 6.9 | 24.2 ± 7.7 | 22.8 ± 6.1 | 0.264 |
| 1 y | 24.6 ± 10.9 | 23.7 ± 6.6 | 23.2 ± 6.2 | 0.453 |
| 2 y | 23.8 ± 6.7 | 25.1 ± 10.1 | 23.0 ± 4.9 | 0.198 |
| Alkaline phosphatase (35–115 U/L) | ||||
| Baseline | 75.1 ± 21.8 | 76.8 ± 20.9 | 74.2 ± 20.0 | 0.597 |
| 1 y | 73.3 ± 19.9 | 76.2 ± 21.7 | 73.8 ± 19.4 | 0.718 |
| 2 y | 73.9 ± 17.4 | 74.3 ± 18.7 | 70.9 ± 17.0 | 0.423 |
| Albumin (3.4–4.8 g/dL) | ||||
| Baseline | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.733 |
| 1 y | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.3 ± 0.3 | 0.562 |
| 2 y | 4.3 ± 0.3 | 4.3 ± 0.3 | 4.2 ± 0.4 | 0.181 |
| Protein (6.3–8.3 g/dL) | ||||
| Baseline | 7.0 ± 0.4 | 7.0 ± 0.5 | 7.1 ± 0.4 | 0.450 |
| 1 y | 7.0 ± 0.4 | 6.9 ± 0.4 | 7.0 ± 0.5 | 0.259 |
| 2 y | 7.0 ± 0.4 | 7.1 ± 0.5 | 7.0 ± 0.5 | 0.264 |
All values are means ± SDs. CBC, complete blood count; WBC, white blood cells; RBC, red blood cells; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; MPV, mean platelet volume; SEG, segmented neutrophils; ANC, absolute neutrophil count; BUN, blood urea nitrogen; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
P values for baseline and years 1 and 2 were derived from a multivariate model ANOVA with adjustment for study site, soy intake, and pretreatment values. The interaction terms were not significant. Number of study participants at baseline (placebo: 134; 80 mg/d: 135; 120 mg/d: 134), after 1 y of treatment (placebo: 128; 80 mg/d: 122; 120 mg/d: 123), and after 2 y of treatment (placebo: 126; 80 mg/d: 119; 120 mg/d: 117).
TABLE 3.
Descriptive statistics for blood lipids and hormones in the 3 treatment groups at baseline and after 1 and 2 y of soy isoflavone supplementation1
| Soy isoflavone–treated groups |
||||
| Variables (normal range) and sequence | Placebo | 80 mg/d | 120 mg/d | 2 |
| Blood lipids | ||||
| Cholesterol (0–200 mg/dL) | ||||
| Baseline | 213.2 ± 32.5 | 218.7 ± 33.4 | 215.4 ± 34.6 | 0.399 |
| 1 y | 211.1 ± 33.8 | 215.9 ± 36.1 | 212.9 ± 37.4 | 0.924 |
| 2 y | 209.0 ± 32.6 | 216.8 ± 35.6 | 213.5 ± 39.5 | 0.787 |
| Triglycerides (35–160 mg/dL) | ||||
| Baseline | 95.2 ± 41.2 | 100.0 ± 57.7 | 97.6 ± 49.2 | 0.729 |
| 1 y | 93.7 ± 42.1 | 99.8 ± 47.5 | 98.5 ± 57.0 | 0.762 |
| 2 y | 102.1 ± 54.2 | 104.3 ± 54.1 | 101.5 ± 68.7 | 0.802 |
| HDL cholesterol (>35 mg/dL) | ||||
| Baseline | 68.1 ± 15.6 | 67.3 ± 16.3 | 68.1 ± 18.1 | 0.905 |
| 1 y | 66.6 ± 14.2 | 65.1 ± 15.4 | 67.0 ± 16.7 | 0.396 |
| 2 y | 64.7 ± 14.6 | 63.1 ± 15.1 | 65.3 ± 16.6 | 0.557 |
| LDL cholesterol (<130 mg/dL) | ||||
| Baseline | 126.1 ± 30.0 | 131.4 ± 30.5 | 127.9 ± 30.9 | 0.359 |
| 1 y | 125.2 ± 30.2 | 130.5 ± 33.5 | 127.0 ± 32.5 | 0.811 |
| 2 y | 123.9 ± 29.3 | 133.6 ± 31.9 | 127.6 ± 34.8 | 0.298 |
| Endocrine profile | ||||
| TSH (0.35–5.5 μIU/mL) | ||||
| Baseline | 2.8 ± 7.4 | 2.2 ± 1.5 | 2.4 ± 2.9 | 0.612 |
| 1 y | 2.6 ± 2.4 | 2.5 ± 1.6 | 2.6 ± 3.2 | 0.850 |
| 2 y | 2.4 ± 1.4 | 2.4 ± 1.3 | 2.2 ± 1.2 | 0.264 |
| FT4 (0.80–1.80 ng/dL) | ||||
| Baseline | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.810 |
| 1 y | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.739 |
| 2 y | 1.2 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.2 | 0.052 |
| Hormone profile (postmenopausal) | ||||
| LH (7.7–58.5 mIU/mL) | ||||
| Baseline | 36.4 ± 11.5 | 37.0 ± 13.9 | 41.8 ± 14.0 | 0.112 |
| 1 y | 36.8 ± 12.4 | 36.7 ± 16.9 | 37.2 ± 13.3 | 0.382 |
| 2 y | 34.5 ± 13.2 | 34.9 ± 19.0 | 41.2 ± 13.7 | 0.490 |
| FSH (23–117 mIU/mL) | ||||
| Baseline | 74.2 ± 24.0 | 76.8 ± 26.7 | 82.2 ± 30.8 | 0.052 |
| 1 y | 72.6 ± 24.8 | 75.0 ± 29.6 | 75.8 ± 30.7 | 0.284 |
| 2 y | 72.0 ± 23.5 | 78.2 ± 30.0 | 80.3 ± 32.4 | 0.365 |
| Estradiol (<10–31 ng/dL) | ||||
| Baseline | 25.1 ± 23.9 | 24.8 ± 22.3 | 27.2 ± 27.5 | 0.685 |
| 1 y | 26.8 ± 29.5 | 25.5 ± 23.8 | 27.6 ± 27.7 | 0.848 |
| 2 y | 24.2 ± 21.7 | 25.0 ± 21.7 | 27.7 ± 26.5 | 0.126 |
All values are means ± SDs. TSH, thyrotropin; FT4, free thyroxine; LH, luteinizing hormone; FSH, follicle-stimulating hormone.
P values for baseline and years 1 and 2 were derived from a multivariate model ANOVA with adjustment for study site, soy intake, and pretreatment values. The interaction terms were not significant. Number of study participants at baseline (placebo: 134; 80 mg/d: 135; 120 mg/d: 134), after 1 y of treatment (placebo: 128; 80 mg/d: 122; 120 mg/d: 123), and after 2 y of treatment (placebo: 126; 80 mg/d: 119; 120 mg/d: 117).
Clinical examination measures
Screening and well-woman examinations over the course of the study did not result in any significant differences in Papanicolau test, stool guaiac test, blood pressure, and screening mammogram results between the treatment groups (data not shown). A review of the organ systems for adverse findings showed predominately insignificant differences. The only outcome that was significantly different between treatment groups was that of musculoskeletal complaints at year 2, when both supplementation groups had a greater number of subjective complaints than did the placebo group (P = 0.027). Although this value was significant, the small numbers of occurrences limit a statistical or clinical interpretation of these data. Baseline data for the subjects by group indicated that 4 of 134 women (2.99%) in the placebo group, 11 of 135 women (8.15%) in the 80-mg isoflavone group, and 6 of 135 women (4.44%) in the 120-mg isoflavone treatment group reported musculoskeletal discomfort. At year 2, the data showed that 1 of 127 women (0.79%) in the placebo group, 9 of 118 women (7.63%) in the 80-mg isoflavone group, and 5 of 116 women (4.31%) in 120-mg isoflavone treatment group reported musculoskeletal discomfort.
Transvaginal ultrasound measurements on 116 women from the California subject cohort showed no significant differences in endometrial thickness between the 3 treatment groups over the course of the study. Baseline and year 2 values by group were, respectively, as follows: placebo (2.0 ± 1.22 and 1.5 ± 1.25 mm), 80 mg isoflavone (1.9 ± 1.67 and 1.3 ± 0.74 mm), and 120 mg isoflavone (1.8 ± 0.98 and 1.0 ± 0.58 mm). Mean values for the total group were 1.9 ± 1.31 mm (baseline), 1.6 ± 0.98 mm (year 1), and 1.3 ± 0.94 mm (year 2). Log transformation of the data and repeat statistical analyses did not change the results. Only 13 women had detectable uterine fibroids (data not shown), and no growth of any uterine fibroids and no statistical differences between the treatment groups over the 2-y interval were observed.
Serious adverse events
Two of the 403 women in the study experienced serious adverse events during the research period; neither subject was an equol producer. Breast cancer (pathology unknown) was diagnosed at 14 mo in one woman who received 120 mg soy isoflavones/d, and stage 1B (grade III) adenocarcinoma of the endometrium (pathology indicated lack of estrogen receptors, which suggested an estrogen-independent malignancy) was diagnosed at 21 mo in one woman who received 80 mg soy isoflavones/d.
DISCUSSION
In the past 15 y there has been great interest in the health benefits of soy, particularly estrogen-like isoflavone components. Collectively, the focus on positive soy and isoflavone RCT data and fear of HT encouraged hope in clinicians and patients alike that soy isoflavones would be a safe option for menopausal women. There is, however, a paucity of data in the literature reporting on the clinical outcomes in relation to the health benefits and potential risks of soy isoflavone supplementation in postmenopausal women. Several reports have highlighted the need for such information in well-controlled, longer-term studies, citing that the current data are insufficient to make definitive conclusions regarding safety (5, 7, 8).
Although the primary outcome measure of the OPUS Study focused on osteoporosis prevention, the major secondary outcome measure was safety. After 2 y of daily soy hypocotyl isoflavone exposure, we only found one biochemical variable with a statistically significant but nonclinical difference from placebo (Tables 2 and 3), and only 2 serious adverse events were detected. Although these findings support the position that isoflavone supplementation during this time period presents minimal health risks and is safe, it should be appreciated that the soy hypocotyl isoflavone preparation used in the OPUS Study has a qualitatively different isoflavone composition than many other research and over-the-counter formulations of isoflavones. Supplements derived from soy hypocotyl, as in the present OPUS Study, contain mostly daidzein (54%) and glycitein (34%) and only 12% genistein. In contrast, supplements based on soy cotyledons contain mostly genistein (50%) and daidzein (45%) and only 5% glycitein. In addition, common over-the-counter “isoflavone” supplements also include those from kudzu (puerarin from the root of Pueraria lobata), the 8-C-glycoside of daidzein that is excreted unchanged in urine and is not converted to equol (32, 33), and those from red clover (the 4′-methyl ethers of daidzein and genistein). Most of the recent studies that have examined the safety of isoflavones have involved genistein and soy cotyledon isoflavones (19, 34, 35) and red clover isoflavones (36). It is, therefore, important to appreciate that a limitation of the current findings is that the clinical outcomes information derived from the OPUS Study cannot be necessarily used to establish the safety profile of the other available isoflavone preparations.
It is reasonable to hypothesize that isoflavones may affect thyroid homeostasis, because it is well known that mammalian estrogens, whether endogenous or exogenous, increase thyroid-binding globulin concentrations in women and result in a compensatory increase in thyroxine output to normal physiologic concentrations (37). Animal studies show that isoflavones can inhibit thyroxine at the thyroid peroxidase step (38); however, the clinical relevance of this action to healthy menopausal women who are presumed to be iodine replete is controversial. Nearly all prior studies that examined the effects of isoflavones on thyroid variables in menopausal women have been conducted with soy protein isolate (SPI) and have shown no clinically significant effect on thyroid function (27, 39–43). Because the subjects in our study did not receive SPI and the protein component of soy may have a physiologic effect, the prior studies that examined the effects on thyroid homeostasis are informative but may not be directly comparable with our data. Little long-term data exist regarding the effects of purified isoflavones on human thyroid function. However, a recent RCT showed that supplementation with genistein aglycone for 3 y did not affect thyroid hormones or autoantibodies in osteopenic menopausal women (44). The current study used a low-genistein supplement. Despite these differences, our study findings are consistent with previous data indicating that isoflavones do not have a clinical effect on thyroid function in healthy menopausal women. Although the changes in serum FT4 concentrations were nearly significant over the 2-y study interval, these changes were clinically insignificant, and all values remained within the normal reference ranges. Lack of changes in clinical chemistry values for circulating serum hormones and lipids in response to purified isoflavones were also consistent with the results of other investigators (45).
Another blood variable that we were keenly interested in was the lymphocyte concentration, because ipriflavone—a synthetic isoflavone studied in a large 2-y RCT—induced lymphocytopenia in a significant number of menopausal subjects, albeit at a very high dose (600 mg/d) (26). Mild neutropenia was also observed in postmenopausal women who ingested a single very high dose (2–16 mg/kg body wt) of purified isoflavones, which reversed on cessation of the supplement (46). Conversely, our findings showed no change in blood lymphocyte concentrations between either the 80- or 120-mg soy hypocotyl treatment groups and the placebo group at either 1 or 2 y. Our results corroborate those of a 1-y trial using SPI (0 mg compared with 60 mg isoflavones) in menopausal women; daily supplementation with soy hypocotyl isoflavones (≤120 mg/d) did not affect lymphocyte concentrations (47).
In our study, there were very few subjective complaints and adverse events noted during the well-women examinations and review of organ systems. The 2 serious adverse events included one subject with endometrial cancer and one subject with breast cancer. Because cancers in these tissues are potentially estrogen sensitive, it is theoretically possible that isoflavones could have a role in their pathogenesis. According to the American Cancer Society, the probability of a woman in the United States aged 40–59 y during 2002–2004 of developing invasive endometrial cancer was 1 in 142 (48). The incidence of endometrial cancer during the OPUS Study, 1 in 403 subjects, was not significantly different from what would be predicted (P = 0.290). Consistent with all prior RCTs that examined the effects of soy isoflavones on the endometrium, with one exception, we observed no effect on endometrial thickness assessed by transvaginal ultrasound (19–22, 49, 50). Additionally, epidemiologic data consistently show that Asian women have nearly one-tenth the risk of endometrial cancer compared with women in Western societies, which is believed to be due, in part, to the influence of soy consumption (51–53). As a point of reference, unopposed traditional (mammalian) estrogen therapy causes endometrial hyperplasia in 30% of menopausal women after 3 y of exposure—a 10-fold difference compared with the one outlier soy isoflavone study (1, 21). It is suggested that simple endometrial hyperplasia leads to cancer in ≈1% of patients (54). Therefore, our data do not support a link between 80 or 120 mg soy hypocotyl isoflavone intake and endometrial cancer risk in healthy menopausal women after 2 y of daily exposure.
No RCTs have examined a link between soy isoflavones and breast cancer as a primary outcome measure, neither increasing nor decreasing risk. In our study, one subject in the 120-mg soy hypocotyl isoflavone supplementation group developed breast cancer 14 mo after enrollment. Given the probability of US women developing breast cancer during our study (1 in 26), the incidence of subjects in our study who developed breast cancer (1 in 403) was statistically lower than that predicted (P < 0.01) (48). Because the metrics of safety were secondary outcomes in the OPUS Study, we could not conclude that soy hypocotyl isoflavone supplementation actually decreases the risk of breast cancer; however, our data are reassuring. Accumulating data in the literature do not indicate that soy or soy isoflavone exposure increases breast cancer risk (55–57). Additional support for this viewpoint from the OPUS population indicates that soy isoflavones do not modify mammographic densities in menopausal women (24).
Soy isoflavones are not biologically inert and may act as weak selective estrogen receptor modulators. Current data in the literature suggest that soy and soy isoflavones may provide a mild benefit to hot flashes, lipids, and bone health for some menopausal women, but we do not yet have the means to identify which women will actually receive the benefits and which will have no benefit. With regard to clinical outcomes, our 2-y data suggest a minimal health risk associated with soy hypocotyl isoflavone supplementation (80–120 mg/d) in healthy menopausal women. Retention of subjects in this long-term study was very good; however, the reduced sample sizes at year 1 and 2 may have diminished the ability to detect significant changes in the secondary outcome variables. However, as noted previously, none of the final values or measurements was found to be outside the established normal ranges. Therefore, we believe that the sample size did not change our conclusion that soy isoflavone supplementation of ≤120 mg/d for 2 y has no adverse effects on the clinical outcomes described in this manuscript. The current study results do not necessarily apply to other soy foods or isoflavone formulations, because other products have different isoflavone profiles. In conclusion, the results of this RCT in 403 menopausal women support the safety of soy hypocotyl isoflavone supplementation over a 2-y period.
Acknowledgments
The authors’ responsibilities were as follows—JKF and EOS: managed the database and performed the statistical analyses; MJM, MAC, RLY, and PA: performed the annual well-woman examinations; and MJM: performed the clinical examinations for endometrial thickness and fibroids. All authors contributed to the study design, execution of the clinical trial, and preparation of the manuscript. SB holds a US patent for the use of conjugated isoflavones in the prevention of osteoporosis. None of the other authors had any conflicts of interest to declare.
REFERENCES
- 1.The Writing Group for the PEPI Trial Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. JAMA 1996;275:370–5 [DOI] [PubMed] [Google Scholar]
- 2.Rossouw JE, Anderson GL, Prentice R, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–33 [DOI] [PubMed] [Google Scholar]
- 3.Woodruff JD, Pickar JH. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. The Menopause Study Group. Am J Obstet Gynecol 1994;170:1213–23 [DOI] [PubMed] [Google Scholar]
- 4.Haimov-Kochman R, Brezinski A, Hochner-Celnikier D. Herbal remedies for menopausal symptoms: are we cautious enough? Eur J Contracept Reprod Health Care 2008;13:133–7 [DOI] [PubMed] [Google Scholar]
- 5.Eisenbrand G. Isoflavones as phytoestrogens in food supplements and dietary foods for special medical purposes. Opinion of the Senate Commission on Food Safety (SKLM) of the German Research Foundation (DFG)-(shortened version). Mol Nutr Food Res 2007;51:1305–12 [DOI] [PubMed] [Google Scholar]
- 6.Wuttke W, Jarry H, Seidlova-Wuttke D. Isoflavones—safe food additives or dangerous drugs? Ageing Res Rev 2007;6:150–88 [DOI] [PubMed] [Google Scholar]
- 7.Federal Institute for Risk Assessment Isolated isoflavones are not without risk. Updated BfR Expert Opinion No. 039/2007. Berlin, Germany: Federal Institute for Risk Assessment, 2007. Available from: http://www.bfr.bund.de/cm/245/isolated_isoflavones_are_not_without_risk.pdf [Google Scholar]
- 8.Song WO, Chun OK, Hwang I, et al. Soy isoflavones as safe functional ingredients. J Med Food 2007;10:571–80 [DOI] [PubMed] [Google Scholar]
- 9.Messina M, Nagata C, Wu AH. Estimated Asian adult soy proteín and isoflavone intakes. Nutr Cancer 2006;55:1–12 [DOI] [PubMed] [Google Scholar]
- 10.van der Schouw YT, Kreijkamp-Kaspers S, Peeters PH, Keinan-Boker L, Rimm EB, Grobbee DE. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in Western women. Circulation 2005;111:465–71 [DOI] [PubMed] [Google Scholar]
- 11.Chandrareddy A, Muneyyirci-Delale O, McFarlane SI, Murad OM. Adverse effects of phytoestrogens on reproductive health: a report of three cases. Complement Ther Clin Pract 2008;14:132–5 [DOI] [PubMed] [Google Scholar]
- 12.Pop EA, Fischer LM, Coan AD, Gitzinger M, Nakamura J, Zeisel SH. Effects of a high daily dose of soy isoflavones on DNA damage, apoptosis, and estrogenic outcomes in healthy postmenopausal women: a phase I clinical trial. Menopause 2008;15:684–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson JW, Johnstone BM, Cook-Newell ME. Meta-analysis of the effects of soy protein intake on serum lipids. N Engl J Med 1995;333:276–82 [DOI] [PubMed] [Google Scholar]
- 14.Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW., Jr Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr 1998;68:1375S–9S [DOI] [PubMed] [Google Scholar]
- 15.Wangen KE, Duncan AM, Xu X, Kurzer MS. Soy isoflavones improve plasma lipids in normocholesterolemic and mildly hypercholesterolemic postmenopausal women. Am J Clin Nutr 2001;73:225–31 [DOI] [PubMed] [Google Scholar]
- 16.Upmalis DH, Lobo R, Bradley L, Warren M, Cone FL, Lamia CA. Vasomotor symptom relief by soy isoflavone extract tablets in postmenopausal women: a multicenter, double blind, randomized, placebo-controlled study. Menopause 2000;7:236–42 [DOI] [PubMed] [Google Scholar]
- 17.Kreijkamp-Kaspers S, Kok L, Grobee DE, et al. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: a randomized controlled trial. JAMA 2004;292:65–74 [DOI] [PubMed] [Google Scholar]
- 18.Atteritano M, Marini H, Minutoli L, et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab 2007;92:3068–75 [DOI] [PubMed] [Google Scholar]
- 19.Marini H, Bitto A, Altavilla D, et al. Breast safety and efficacy of genistein aglycone for postmenopausal bone loss: a follow-up study. J Clin Endocrinol Metab 2008;93:4787–96 [DOI] [PubMed] [Google Scholar]
- 20.Palacios S, Pornel B, Bergeron C, et al. Endometrial safety assessment of a specific and standardized soy extract according to international guidelines. Menopause 2007;14:1006–11 [DOI] [PubMed] [Google Scholar]
- 21.Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo- controlled study. Fertil Steril 2004;82:145–8 [DOI] [PubMed] [Google Scholar]
- 22.Alekel DL, Van Loan MD, Koehler KJ, et al. The soy isoflavones for reducing bone loss (SIRBL) study: a 3-y randomized controlled trial in postmenopausal women. Am J Clin Nutr 2010;91:218–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong WW, Lewis RD, Steinberg FM, et al. Soy isoflavones supplementation and bone mineral density in menopausal women: a two year multicenter clinical trial. Am J Clin Nutr 2009;90:1433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maskarinec G, Verheus M, Steinberg FM, et al. Various doses of soy isoflavones do not modify mammographic density in postmenopausal women. J Nutr 2009;139:981–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Obstetricians and Gynecologists Guidelines for women's health care: a resource manual. 3rd ed Washington, DC: American College of Obstetricans and Gynecologists, 2007 [Google Scholar]
- 26.Alexandersen P, Toussaint A, Christiansen C, et al. Ipriflavone in the treatment of postmenopausal osteoporosis: a randomized controlled trial. JAMA 2001;285:1482–8 [DOI] [PubMed] [Google Scholar]
- 27.Duncan AM, Underhill KE, Xu X, Lavalleur J, Phipps WR, Kurzer MS. Modest hormonal effects of soy isoflavones in postmenopausal women. J Clin Endocrinol Metab 1999;84:3479–84 [DOI] [PubMed] [Google Scholar]
- 28.Song T, Barua K, Buseman G, Murphy PA. Soy isoflavone analysis: quality control and a new internal standard. Am J Clin Nutr 1998;68(suppl):1474S–9S [DOI] [PubMed] [Google Scholar]
- 29.Coward L, Kirk M, Albin N, Barnes S. Analysis of plasma isoflavones by reversed-phase HPLCC-multiple reaction ion monitoring-mass spectrometry. Clin Chim Acta 1996;29:247(1–2):121–42 [DOI] [PubMed] [Google Scholar]
- 30.Hanley JA, Negassa A, Edwardes MD, Forrester JE. Statistical analysis of correlated data using generalized estimating equations: an orientation. Am J Epidemiol 2003;157:364–75 [DOI] [PubMed] [Google Scholar]
- 31.Setchell KD, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr 2006;136:2188–93 [DOI] [PubMed] [Google Scholar]
- 32.Prasain JK, Jones K, Kirk M, et al. Profiling and quantification of isoflavonoids in kudzu dietary supplements by high- performance liquid chromatography and electrospray ionization tandem mass spectrometry. J Agric Food Chem 2003;51:4213–8 [DOI] [PubMed] [Google Scholar]
- 33.Prasain JK, Jones K, Brissie N, Moore R, Wyss JM, Barnes S. Identification of puerarin and its metabolites in rats by liquid chromatography-tandem mass spectrometry. J Agric Food Chem 2004;52:3708–12 [DOI] [PubMed] [Google Scholar]
- 34.Gleason CE, Carlsson CM, Barnet JH, et al. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing 2009;38:86–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nahas EA, Nahas-Neto J, Orsatti FL, Carvalho EP, Oliveira ML, Dias R. Efficacy and safety of a soy isoflavone extract in postmenopausal women: a randomized, double- blind, and placebo-controlled study. Maturitas 2007;58:249–58 [DOI] [PubMed] [Google Scholar]
- 36.Powles TJ, Howell A, Evans DG, et al. Red clover isoflavones are safe and well tolerated in women with a family history of breast cancer. Menopause Int 2008;14:6–12 [DOI] [PubMed] [Google Scholar]
- 37.Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 38.Doerge DR, Sheehan DR. Goitrogenic and estrogenic activity of soy isoflavones. Environ Health Perspect 2002;110(suppl 31):349–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bruce B, Messina M, Spiller GA. Isoflavone supplements do not affect thyroid function in iodine-replete postmenopausal women. J Med Food 2003;6:309–16 [DOI] [PubMed] [Google Scholar]
- 40.Messina M, Redmond G. Effects of soy protein and soybean isoflavones on thyroid function in healthy adults and hypothyroid patients: a review of the relevant literature. Thyroid 2006;16:249–58 [DOI] [PubMed] [Google Scholar]
- 41.Persky VW, Turyk ME, Wang L, et al. Effect of soy protein on endogenous hormones in postmenopausal women. Am J Clin Nutr 2002;75:145–53 [DOI] [PubMed] [Google Scholar]
- 42.Teas J, Braverman LE, Kurzer MS, Pino S, Hurley TG, Hebert JR. Seaweed and soy: companion foods in Asian cuisine and their effects on thyroid function in American women. J Med Food 2007;10:90–100 [DOI] [PubMed] [Google Scholar]
- 43.Teede HJ, Dalais FS, McGrath BP. Dietary soy containing phytoestrogens does not have detectable estrogenic effects on hepatic protein synthesis in postmenopausal women. Am J Clin Nutr 2004;79:396–401 [DOI] [PubMed] [Google Scholar]
- 44.Bitto A, Polito F, Atteritano M, et al. Genistein aglycone does not affect thyroid function: results from a three-year, randomized double-blind, placebo-controlled trial. J Clin Endocrinol Metab 2010;95(6):3067–72 [DOI] [PubMed] [Google Scholar]
- 45.Hooper L, Ryder JJ, Kurzer MS, et al. Effects of soy protein and isoflavones on circulating hormone concentrations in pre-and post-menopausal women: a systematic review and meta-analysis. Hum Reprod Update 2009;15:423–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloedon LT, Jeffcoat AR, Lopaczynski W, et al. Safety and pharmacokinetics of purified soy isoflavones: single-dose administration to postmenopausal women. Am J Clin Nutr 2002;76:1126–37 [DOI] [PubMed] [Google Scholar]
- 47.Soung do Y, Patade A, Khalil DA, et al. Soy protein supplementation does not cause lymphocytopenia in postmenopausal women. Nutr J 2006;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Cancer Society Cancer facts & figures 2008. Atlanta, GA: American Cancer Society, 2008 [Google Scholar]
- 49.Murray MJ, Meyer WR, Lessey BA, Oi RH, DeWire RE, Fritz MA. Soy protein isolate with isoflavones does not prevent estradiol-induced endometrial hyperplasia in postmenopausal women: a pilot trial. Menopause 2003;10:456–64 [DOI] [PubMed] [Google Scholar]
- 50.Foth D, Nawroth F. Effect of phytoestrogens on the endometrium? Fertil Steril 2005;83:256–7 [DOI] [PubMed] [Google Scholar]
- 51.Wynder EL, Fujita Y, Harris RE, Hirayama T, Hiyama T. Comparative epidemiology of cancer between the United States and Japan. A second look. Cancer 1991;67:746–63 [DOI] [PubMed] [Google Scholar]
- 52.Burke TW, Tortolero-Luna G, Malpica A, et al. Endometrial hyperplasia and endometrial cancer. Obstet Gynecol Clin North Am 1996;23:411–56 [PubMed] [Google Scholar]
- 53.Hale GE, Hughes CL, Cline JM. Endometrial cancer: hormonal factors, the perimenopausal “window of risk”, and isoflavones. J Clin Endocrinol Metab 2002;87:3–15 [DOI] [PubMed] [Google Scholar]
- 54.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer 1985;56:403–12 [DOI] [PubMed] [Google Scholar]
- 55.Messina M, Wu AH. Perspectives on the soy-breast cancer relation. Am J Clin Nutr 2009;89(suppl):1673S–9S [DOI] [PubMed] [Google Scholar]
- 56.Wu AH, Yu MC, Tseng C-C, Pike MC. Epidemiology of soy exposure and breast cancer risk. Br J Cancer 2008;98:9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor CK, Levey RM, Elliott JC, Burnett BP. The effect of genistein aglycone on cancer and cancer risk: a review of in vitro, preclinical, and clinical studies. Nutr Rev 2009;67:398–415 [DOI] [PubMed] [Google Scholar]