Abstract
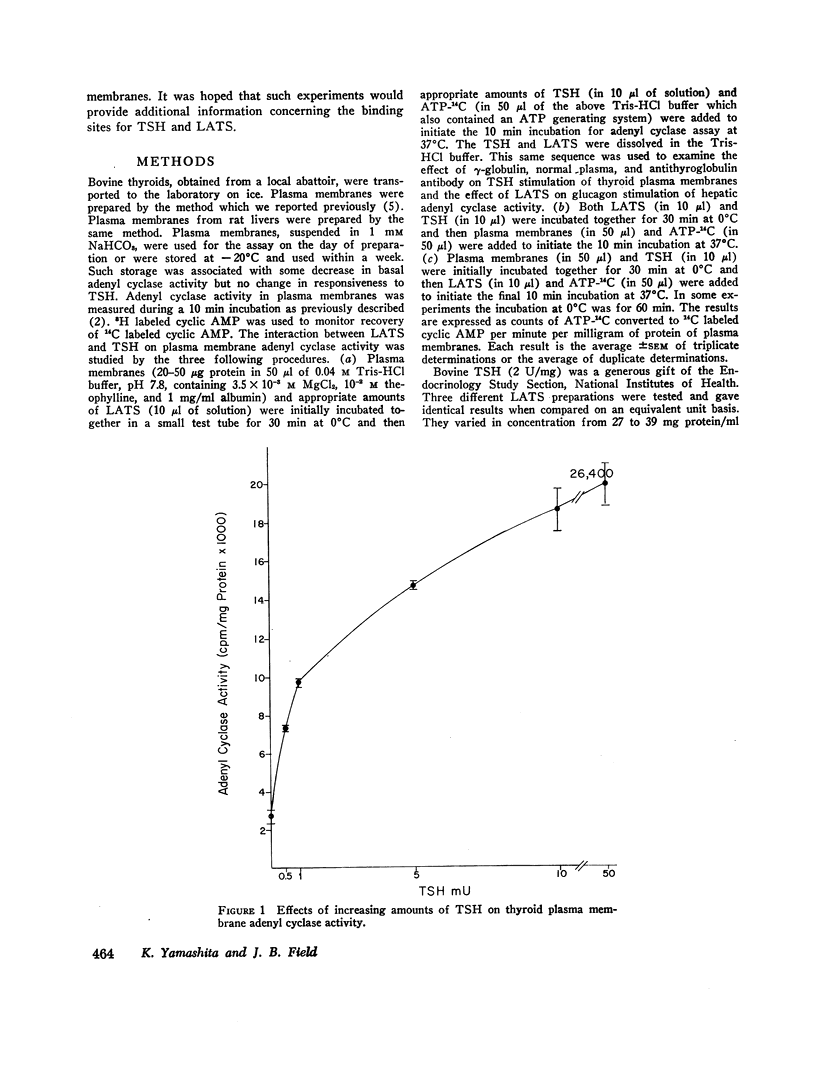
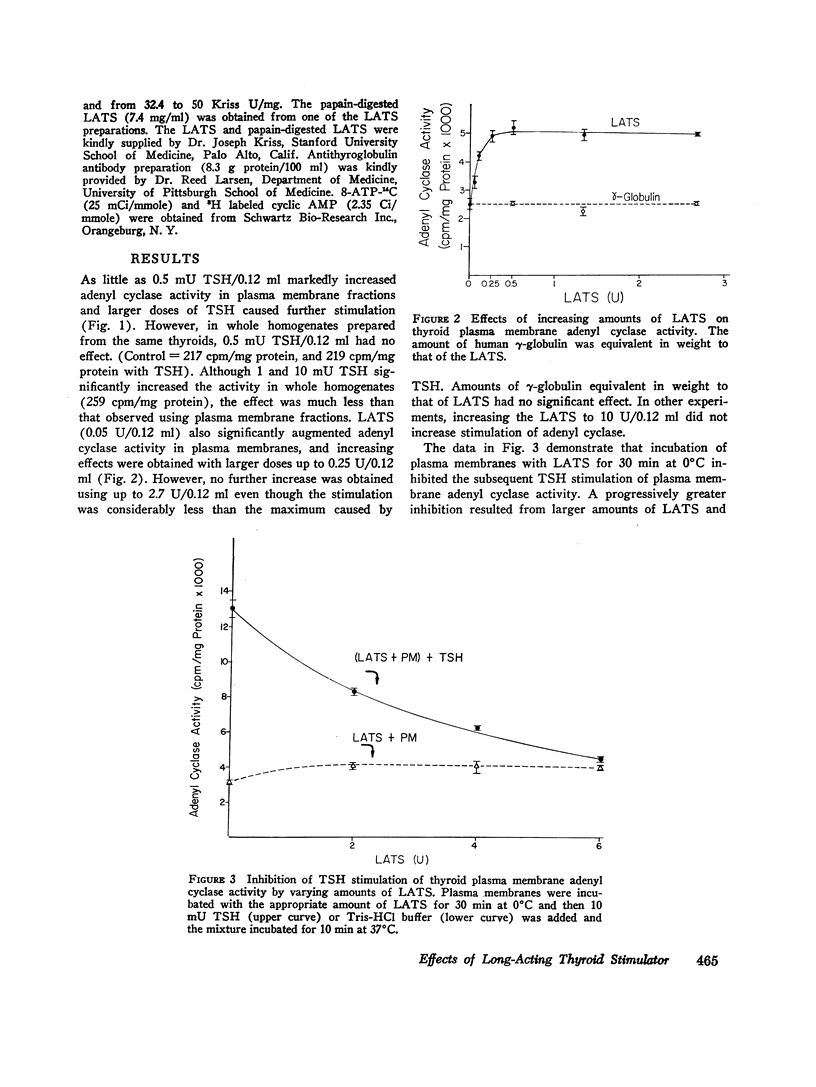
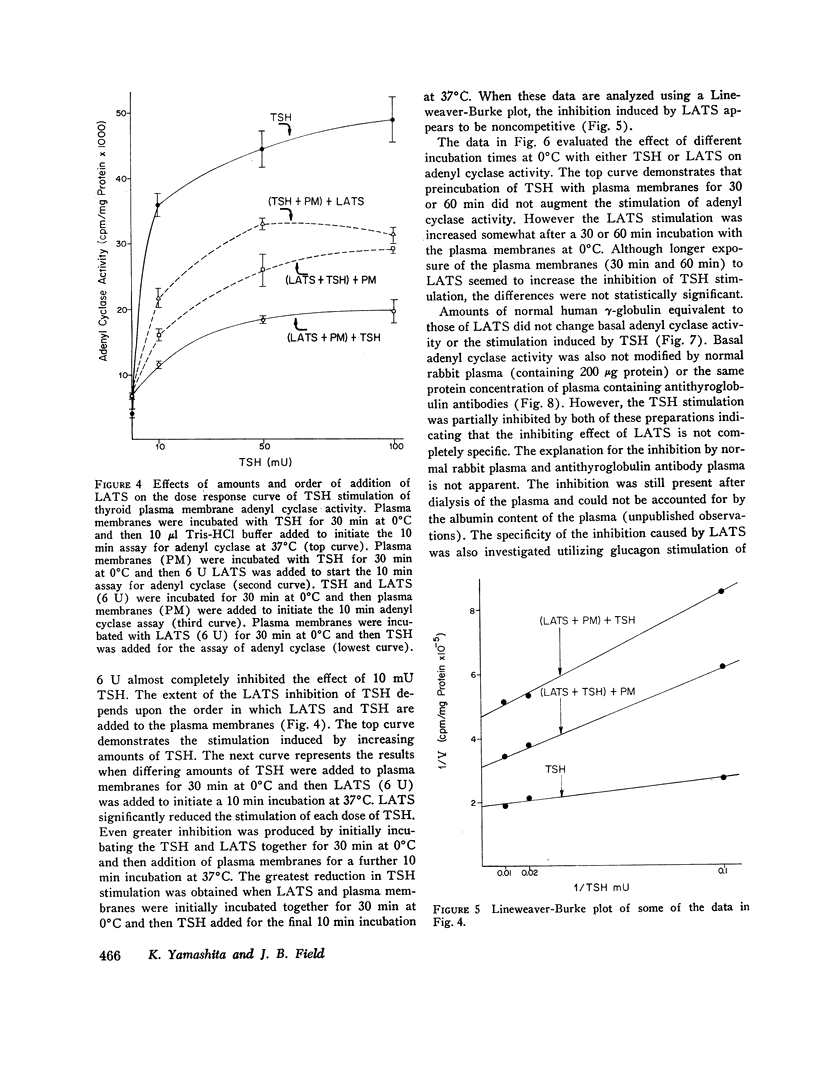
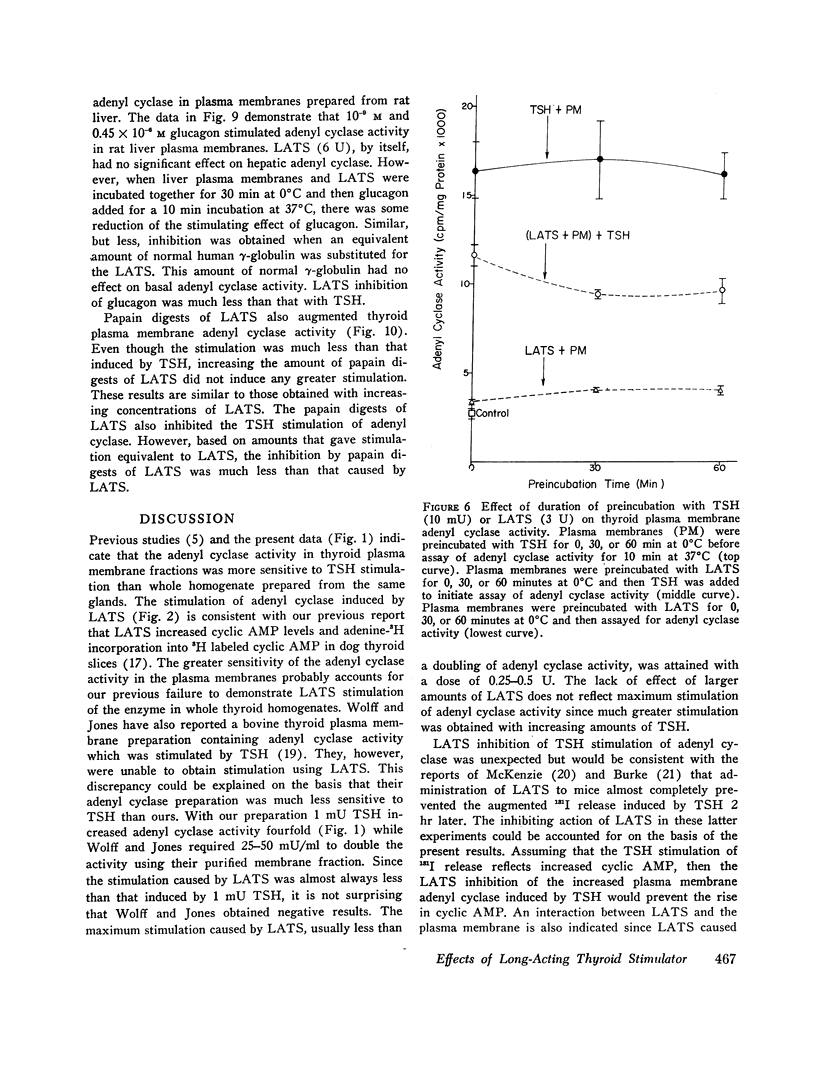
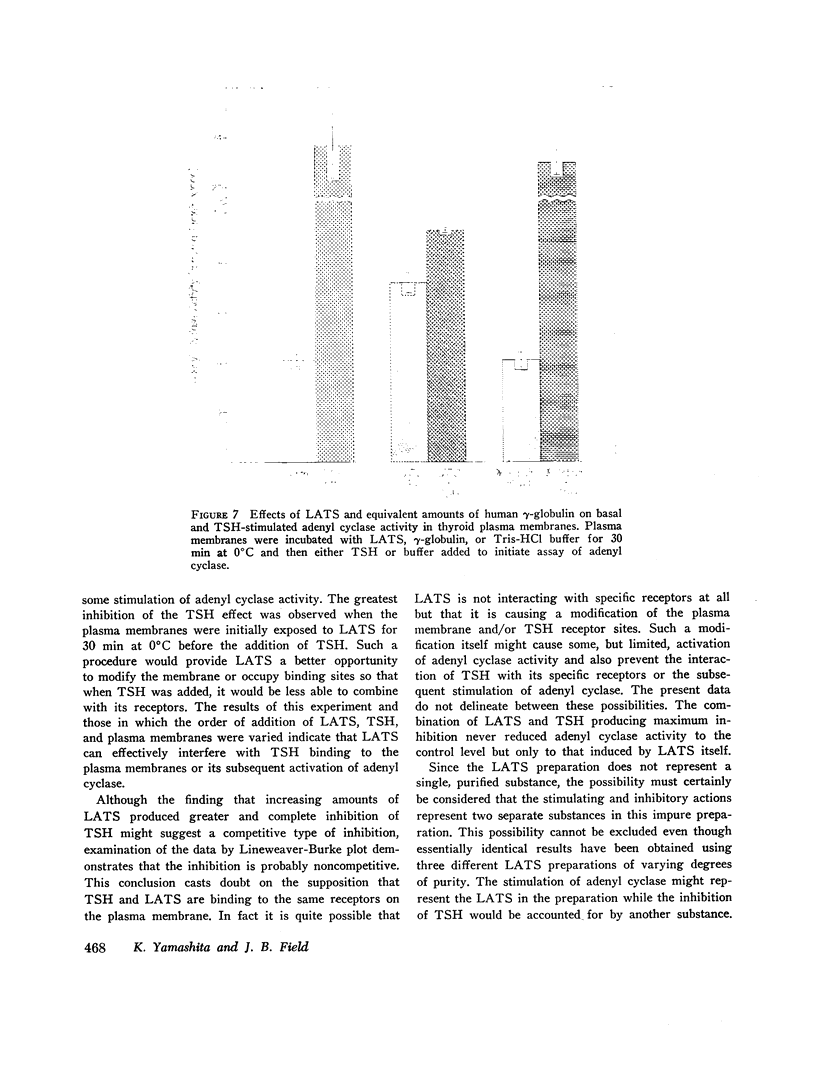
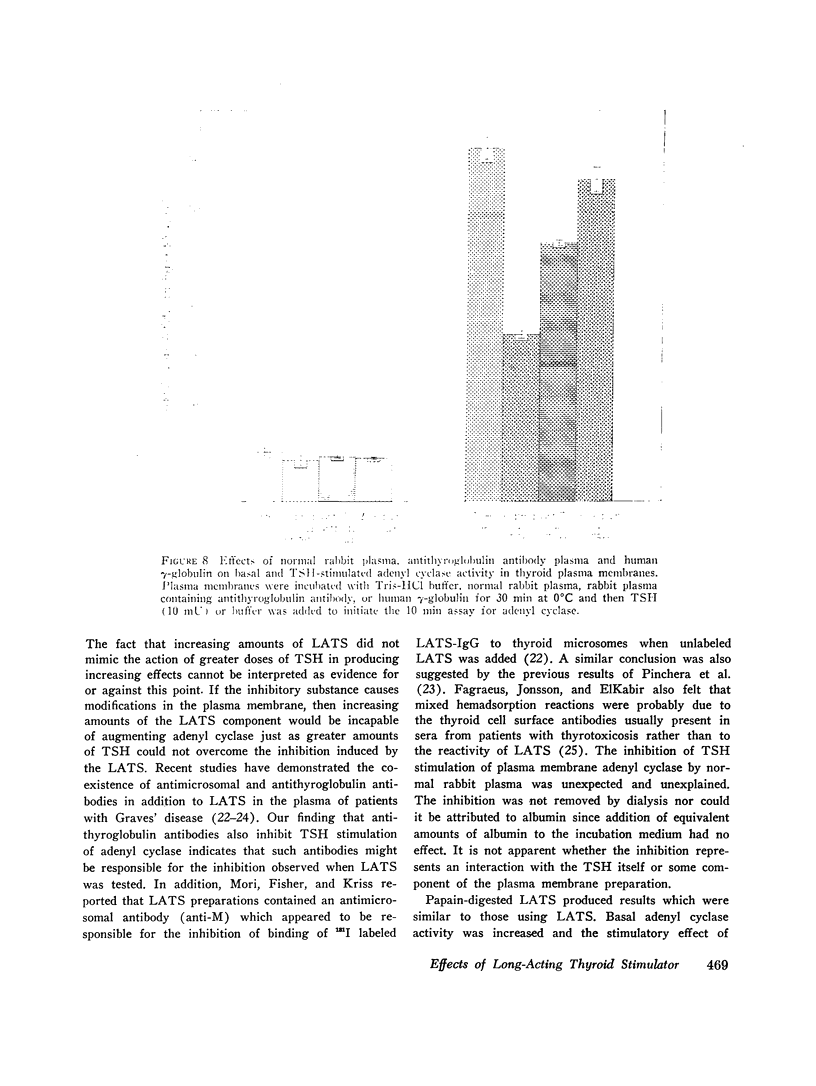
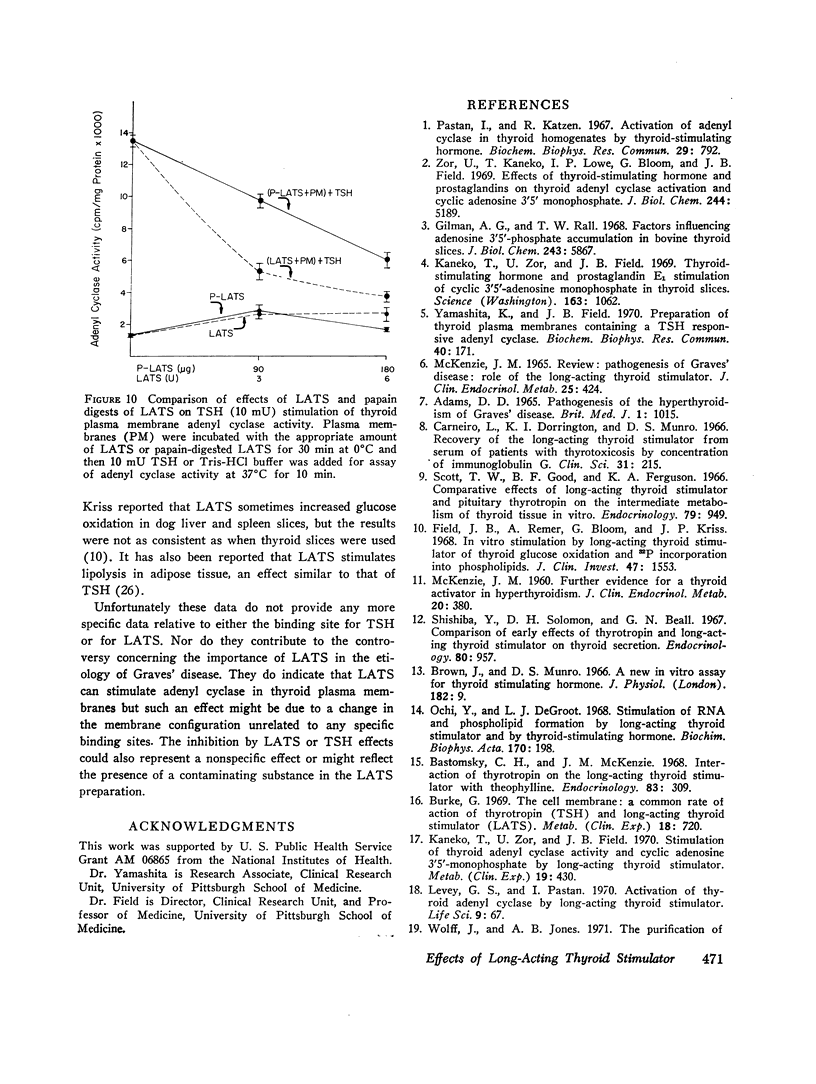
Both thyroid-stimulating hormone (TSH) and long-acting thyroid stimulator (LATS) stimulated adenyl cyclase activity in plasma membranes obtained from bovine thyroid glands. The stimulation induced by LATS was much less than that obtained with maximal amounts of TSH. LATS inhibited TSH stimulation of adenyl cyclase activity while an equivalent amount of normal human γ-globulin did not influence basal or TSH-stimulated activity. The inhibition by LATS appeared to be noncompetitive and was greatest when the plasma membranes were initially exposed to LATS for 30 min at 0°C before being incubated with TSH for 10 min at 37°C. Inhibition could still be demonstrated when the plasma membranes were incubated for 30 min at 0°C with TSH before the addition of LATS. Prolonging the period of incubation of plasma membranes with LATS from 30 to 60 min did not augment the stimulation of adenyl cyclase or increase the inhibition of the effect of TSH. Papain digests of LATS also increased adenyl cyclase activity of thyroid plasma membrane and inhibited the stimulation induced by TSH. The inhibitory effect of LATS was not completely specific for TSH and thyroid plasma membranes since glucagon stimulation of adenyl cyclase in hepatic plasma membranes was also inhibited, but to a lesser extent. In contrast to the results obtained with thyroid plasma membranes, LATS did not influence basal adenyl cyclase activity in hepatic plasma membranes. Furthermore equivalent amounts of normal human γ-globulin also decreased glucagon stimulation of adenyl cyclase activity in plasma membranes obtained from liver. The present data suggest that LATS stimulation of adenyl cyclase in thyroid plasma membranes might be due to a change in the membrane configuration rather than binding to a specific receptor site. Such modification of the membrane structure could interfere with the binding of TSH to specific receptors or to the subsequent stimulation of adenyl cyclase. However, the results do not exclude the possibility that some component in the preparation other than LATS might be responsible for the inhibition of the stimulation by TSH.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS D. D. PATHOGENESIS OF THE HYPERTHYROIDISM OF GRAVES'S DISEASE. Br Med J. 1965 Apr 17;1(5441):1015–1019. doi: 10.1136/bmj.1.5441.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastomsky C. H., McKenzie J. M. Interaction of thyrotropin or the long-acting thyroid stimulator with theophylline. Endocrinology. 1968 Aug;83(2):309–313. doi: 10.1210/endo-83-2-309. [DOI] [PubMed] [Google Scholar]
- Bonnyns M., Vanhaelst L. Relationship between the long-acting thyroid stimulator and the thyroid microsomal antibody. J Endocrinol. 1969 Jan;43(1):125–126. doi: 10.1677/joe.0.0430125. [DOI] [PubMed] [Google Scholar]
- Burke G. On the competitive interaction of long-acting thyroid stimulator and thyrotropin in vivo. J Clin Endocrinol Metab. 1968 Feb;28(2):286–293. doi: 10.1210/jcem-28-2-286. [DOI] [PubMed] [Google Scholar]
- Burke G. The cell membrane: a common site of action of thyrotropin (TSH) and long-acting thyroid stimulator (LATS). Metabolism. 1969 Aug;18(8):720–729. doi: 10.1016/0026-0495(69)90087-0. [DOI] [PubMed] [Google Scholar]
- Carneiro L., Dorrington K. J., Munro D. S. Recovery of the long-acting thyroid stimulator from serum of patients with thyrotoxicosis by concentration of immunoglobulin G. Clin Sci. 1966 Oct;31(2):215–221. [PubMed] [Google Scholar]
- Fagraeus A., Jonsson J., el-Kabir D. J. What is the antibody specificity of LATS? J Clin Endocrinol Metab. 1970 Oct;31(4):445–447. doi: 10.1210/jcem-31-4-445. [DOI] [PubMed] [Google Scholar]
- Field J. B., Remer A., Bloom G., Kriss J. P. In vitro stimulation by long-acting thyroid stimulator of thyroid glucose oxidation and 32P incorporation into phospholipids. J Clin Invest. 1968 Jul;47(7):1553–1560. doi: 10.1172/JCI105847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G., Rall T. W. Factors influencing adenosine 3',5'-phosphate accumulation in bovine thyroid slices. J Biol Chem. 1968 Nov 25;243(22):5867–5871. [PubMed] [Google Scholar]
- Hart I. R., McKenzie J. M. Comparison of the effects of thyrotropin and the long-acting thyroid stimulator on guinea pig adipose tissue. Endocrinology. 1971 Jan;88(1):26–30. doi: 10.1210/endo-88-1-26. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Zor U., Field J. B. Stimulation of thyroid adenyl cyclase activity and cyclic adenosine 3',5'-monophosphate by long-acting thyroid stimulator. Metabolism. 1970 Jun;19(6):430–438. doi: 10.1016/0026-0495(70)90094-6. [DOI] [PubMed] [Google Scholar]
- Kaneko T., Zor U., Field J. B. Thyroid-stimulating hormone and prostaglandin E1 stimulation of cyclic 3',5'-adenosine monophosphate in thyroid slices. Science. 1969 Mar 7;163(3871):1062–1063. doi: 10.1126/science.163.3871.1062. [DOI] [PubMed] [Google Scholar]
- Levey G. S., Pastan I. Activation of thyroid adenyl cyclase by long-acting thyroid stimulator. Life Sci I. 1970 Jan 15;9(2):67–73. doi: 10.1016/0024-3205(70)90018-4. [DOI] [PubMed] [Google Scholar]
- MCKENZIE J. M. REVIEW: PATHOGENESIS OF GRAVES' DISEASE: ROLE OF THE LONG-ACTING THYROID STIMULATOR. J Clin Endocrinol Metab. 1965 Mar;25:424–461. doi: 10.1210/jcem-25-3-424. [DOI] [PubMed] [Google Scholar]
- Mori T., Fisher J., Kriss J. P. Studies of an in vitro binding reaction between thyroid microsomes and long acting thyroid stimulator globulin (LATS). I. Development of solid-state competitive binding radioassay methods for measurement of antimicrosomal and antithyroglobuin antibodies. J Clin Endocrinol Metab. 1970 Aug;31(2):119–133. doi: 10.1210/jcem-31-2-119. [DOI] [PubMed] [Google Scholar]
- Ochi Y., DeGroot L. J. Stimulation of RNA and phospholipid formation by long acting thyroid stimulator and by thyroid-stimulating hormone. Biochim Biophys Acta. 1968 Nov 12;170(1):198–201. doi: 10.1016/0304-4165(68)90173-6. [DOI] [PubMed] [Google Scholar]
- Pastan I., Katzen R. Activation of adenyl cyclase in thyroid homogenates by thyroid-stimulating hormone. Biochem Biophys Res Commun. 1967 Dec 29;29(6):792–798. doi: 10.1016/0006-291x(67)90289-6. [DOI] [PubMed] [Google Scholar]
- Pinchera A., Liberti P., De Santis R., Grasso L., Martino E., Baschieri L. Relationship between the long-acting thyroid stimulator and circulating thyroid antibodies in Graves' disease. J Clin Endocrinol Metab. 1967 Dec;27(12):1758–1760. doi: 10.1210/jcem-27-12-1758. [DOI] [PubMed] [Google Scholar]
- Scott T. W., Good B. F., Ferguson K. A. Comparative effects of long-acting thyroid stimulator and pituitary thyrotropin on the intermediate metabolism of thyroid tissue in vitro. Endocrinology. 1966 Nov;79(5):949–954. doi: 10.1210/endo-79-5-949. [DOI] [PubMed] [Google Scholar]
- Shishiba Y., Solomon D. H., Beall G. N. Comparison of early effects of thyrotropin and long-acting thyroid stimulator on thyroid secretion. Endocrinology. 1967 May;80(5):957–961. doi: 10.1210/endo-80-5-957. [DOI] [PubMed] [Google Scholar]
- Wolff J., Jones A. B. The purification of bovine thyroid plasma membranes and the properties of membrane-bound adenyl cyclase. J Biol Chem. 1971 Jun 25;246(12):3939–3947. [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Preparation of thyroid plasma membranes containing a TSH-responsive adenyl cyclase. Biochem Biophys Res Commun. 1970 Jul 13;40(1):171–178. doi: 10.1016/0006-291x(70)91062-4. [DOI] [PubMed] [Google Scholar]
- Zor U., Kaneko T., Lowe I. P., Bloom G., Field J. B. Effect of thyroid-stimulating hormone and prostaglandins on thyroid adenyl cyclase activation and cyclic adenosine 3',5',-monophosphate. J Biol Chem. 1969 Oct 10;244(19):5189–5195. [PubMed] [Google Scholar]