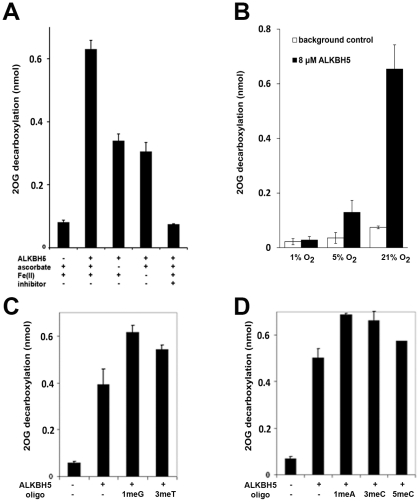
Figure 2. ALKBH5 displays biochemical characteristics of a functional 2OG-dependent oxygenase in vitro.
(A) Enzyme-dependent stimulation of uncoupled turnover that was dependent on the cofactors ascorbate, and Fe(II) and inhibited by 1 mM of the generic 2OG oxygenase inhibitor pyridine-2,4-dicarboxylate (2,4-PDCA). (B) Enzyme-dependent stimulation of uncoupled 2OG turnover that was sensitive to the oxygen concentration. Turnover was significantly reduced under moderate hypoxia (5.0% oxygen) and negated under more severe hypoxia (1.0%) (C) Testing of potential oligonucleotide substrates; 1-methylguanine (1meG) 3-methylthymine (3meT) and (D) 1-methyladenine (1meA), 3-methylcytosine (3meC), 5-methylcytosine (5meC). No significant increase in ALKBH5 activity was observed in the presence of methylated oligonucleotides.