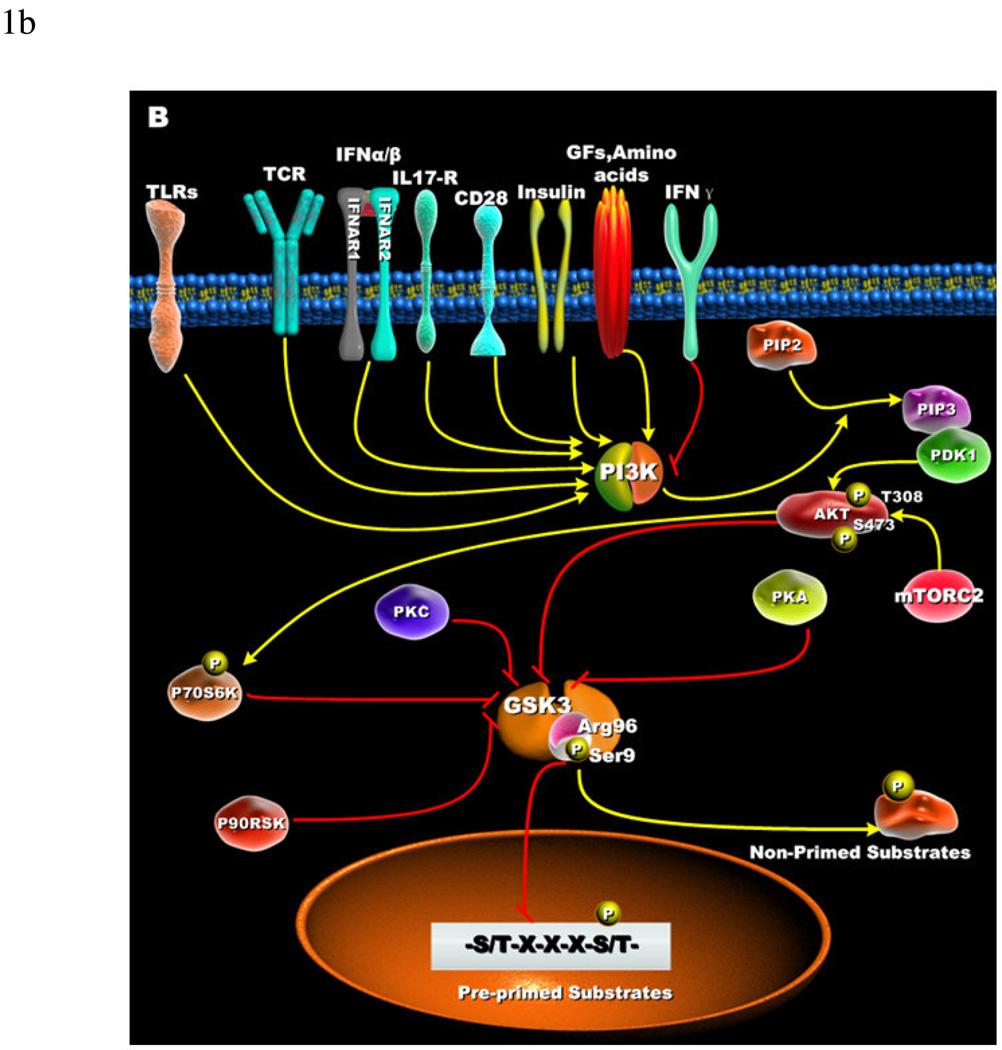
Figure 1. Cellular receptors involved in the phospho-inactivation of GSK3 and substrate specificity.
(A) Growth factors, amino acids, TLRs, TCR, CD28, and cytokine receptors have been shown to mediate the phospho-inactivation of GSK3-α (Ser21) and GSK3-β (Ser9). Activation of PI3K results in the generation of PIP3 that allows for the recruitment of Akt via its pleckstrin homology domain. Full activation of Akt occurs when phosphorylated at threonine 308 by PDK1 and serine 473 by mTORC2. Upon activation, Akt can phosphorylate GSK3-β (Ser9) that results in its inactivation. PKC, p70S6K, p90RSK, and PKA can phospho-inactivate GSK3. Inactivation of GSK3 results in the activation of transcription factors important for regulating the innate and adaptive inflammatory responses. (B) Active GSK3 exhibits a 100- to 1000-fold increase in substrate specificity for pre-primed substrates, as compared to non-primed substrates. N-terminal phosphorylation of GSK3-β (Ser9) acts as a pseudo-substrate for the phosphate-binding site and thus competes for the binding to arginine 96 with pre-primed, but not non-primed substrates.