Figure 3.
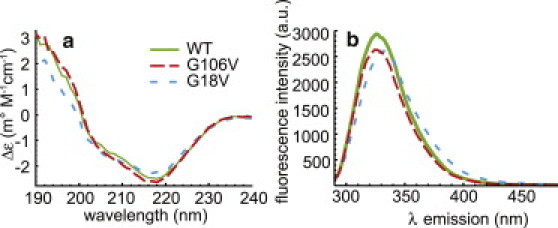
(a) CD spectra of the WT, G18V, and G106V crystallins at a concentration of 0.125 mg/mL in a solution of 10 mM phosphate buffer (pH 6.9) at 20°C. All three display the negative ellipticity at 218 nm that is indicative of β-sheet secondary structure and common to γ-crystallins. (b) Fluorescence emission spectra of WT, G106V, and G18V, 0.25 mg/mL in 10 mM phosphate buffer pH 6.9 at 22°C, collected on a Hitachi F4500 fluorescence spectrophotometer. Both the WT and the G106V variant have a maximum at 326 nm, whereas in the G18V variant it is slightly shifted to 332 nm. Both of these methods indicate little structural difference between the WT and variant proteins at room temperature.
