Figure 1.
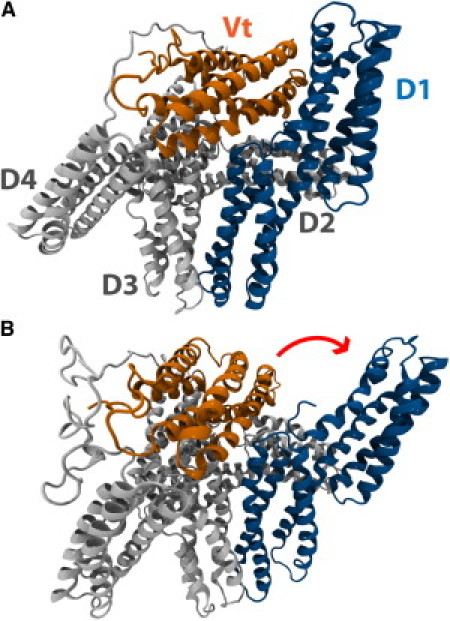
Vinculin can adopt an autoinhibited conformation or an activated conformation. (A) In its native state, vinculin is in an autoinhibited conformation. Vinculin has five helical domains: D1, D2, D3, D4, and Vt. Vt contains binding sites for F-actin whereas D1 contains binding sites for VBS. In its native conformation, the proximity of D1 to Vt prevents the binding of Vt to F-actin. The proximity of Vt to D1 could also impact the binding of D1 to VBS. (B) A suggested conformation of activated vinculin. Investigation of vinculin activation by a stretching force (46) using molecular dynamics has suggested that, during activation, D1 of vinculin undergoes a conformational change and rotates away from Vt. In this conformation, the proximity of Vt and D1 is reduced, potentially allowing for interaction of those domains with binding partners.
