Figure 2. Estrogen significantly enhances the phosphorylation of exogenous LIMK1.
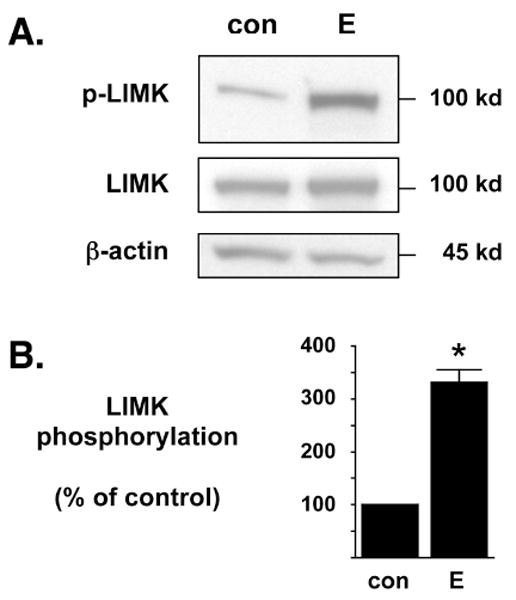
(A) NG108-15 cells were transfected to exogenously express chimeric LIMK1-eGFP and then treated with control (con) or 17β-estradiol (E) for 6 hr. The recombinant LIMK1-eGFP protein (100 kD) is readily discernable from endogenous LIMK protein (72 kD) due to the chimerically attached eGFP marker. In Western blot analyses, the anti-phospho-LIMK antibody is more sensitive to exogenously expressed LIMK protein than to endogenous LIMK protein. Western blot analyses demonstrated that E significantly enhances the phosphorylation of exogenously expressed LIMK1-eGFP (p-LIMK) but does not alter the overall total expression of LIMK1-eGFP (LIMK). As an additional control, the whole cell lysates were also blotted against β-actin protein levels to demonstrate no change in overall endogenous protein levels. (B) Densitometric analyses of immunoblots in (A) demonstrate that estrogen significantly increases the phosphorylation of exogenous LIMK1 when compared to diluent control. The level of phosphorylated LIMK1-eGFP was normalized to the level of total LIMK1-eGFP and densitometry is represented as % of control. *Statistically significant difference between groups (p<0.01), n=5 each.
