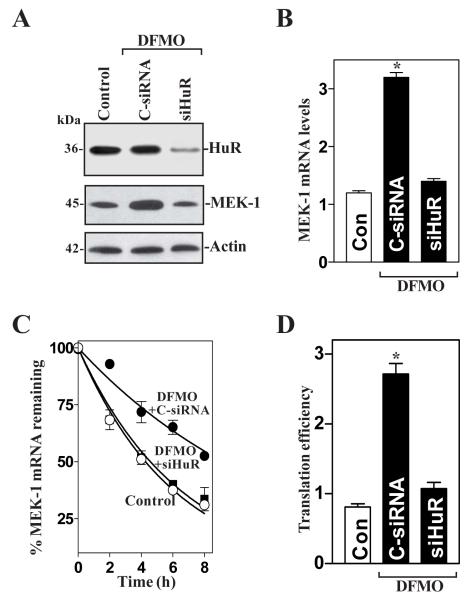
Fig. 5.
Effect of HuR silencing on MEK-1 mRNA stability and its translation in polyamine-deficient cells. (A) Representative HuR and MEK-1 immunoblots. After cells were cultured in the presence of DFMO for 4 days, they were transfected with either siRNA targeting the HuR mRNA coding region (siHuR) or control siRNA (C-siRNA), and whole-cell lysates were harvested 48 h thereafter. The levels of HuR and MEK-1 proteins were measured by Western blot anaylsis, and equal loading was monitored by β-actin immunoblotting. (B) Levels of MEK-1 mRNA in cells that were processed as described in panel A. Total RNA from each group was harvested, and levels of MEK-1 mRNA were measured by RT + Q-PCR analysis. The data were normalized to the amount of GAPDH mRNA and the values represented as the means ± SE of data from triplicate experiments. * P < 0.05 compared with controls and cells transfected with C-siRNA. (C) Half-life of the MEK-1 mRNA in cells that were transfected and treated as described in A. Total cellular RNA was isolated at the indicated times after administration of actinomycin D, and the remaining levels of MEK-1 and GAPDH mRNAs were measured by RT + Q-PCR analysis. Values are means ± SE from triplicate samples. (D) Changes in MEK-1 translation efficiency as measured by using pGL3-Luc-MEK1ARE reporter assays in cells described in A. The pGL3-Luc-MEK1ARE or pGL3-Luc (negative control) was cotransfected with a Renilla luciferase reporter, and firefly and Renilla luciferase activities were assayed 24 h thereafter. Luciferase values were normalized to the mRNA levels to obtain translation efficiencies and expressed as means ± SE of data from 3 separate experiments. * p < 0.05 compared with control cells and DFMO-treated cells transfected with siHuR.