Abstract
Background
Mesenchymal stromal cells have been recently isolated from thymus gland tissue discarded after surgical procedures. The role of this novel cell type in heart regeneration has yet to be defined. The purpose of this study was to evaluate the therapeutic potential of human thymus-derived mesenchymal stromal cells using self-organized cardiac tissue as an in vitro platform for quantitative assessment.
Methods
Mesenchymal stromal cells were isolated from discarded thymus tissue from neonates undergoing heart surgery and were incubated in differentiation media to demonstrate multipotency. Neonatal rat cardiomyocytes self-organized into cardiac tissue fibers in a custom culture dish either alone or in combination with varying numbers of mesenchymal stromal cells. A transducer measured force generated by spontaneously contracting self-organized cardiac tissue fibers. Work and power outputs were calculated from force tracings. Immunofluorescence was performed to determine the fate of the thymus-derived mesenchymal stromal cells.
Results
Mesenchymal stromal cells were successfully isolated from discarded thymus tissue. After incubation in differentiation media, mesenchymal stromal cells attained the expected phenotypes. Although mesenchymal stromal cells did not differentiate into mature cardiomyocytes, addition of these cells increased the rate of fiber formation, force production, and work and power outputs. Self-organized cardiac tissue containing mesenchymal stromal cells acquired a defined microscopic architecture.
Conclusions
Discarded thymus tissue contains mesenchymal stromal cells, which can augment force production and work and power outputs of self-organized cardiac tissue fibers by several-fold. These findings indicate the potential utility of mesenchymal stromal cells in treating heart failure.
Nearly 5 million people have heart failure in the United States, and it currently causes more than 300,000 deaths in adults each year. This prevalence is expected to double by the year 2040 [1]. Furthermore, up to 25% of patients with complex congenital heart disease will eventually experience heart failure [2]. Because the current treatment options for heart failure have many limitations and adverse affects, there is great motivation to develop alternative strategies to treat heart failure.
Mesenchymal stromal cells (MSCs) are adult stem cells that have been demonstrated to have regenerative properties in a number of scenarios of tissue injury. The potential of cell therapy with MSCs has been investigated for heart failure. Numerous in vitro investigations and animal studies based primarily on coronary artery ligation have shown positive, beneficial results and have formed the basis for subsequent human trials [3, 4].
The results in completed human trials have varied; however, meta-analyses of clinical trials using bone marrow–derived stem cells have revealed a small, but significant, improvement in objective measures of cardiac function [5, 6]. The varied results of these clinical trials in heart regenerative therapy arise from a fundamental gap in our knowledge of the optimal stem cell type, dose, route of administration, and timing of therapy [5]. In an effort to eliminate some of the variation inherent in the in vivo studies and to gain more understanding of the true stem cell–mediated effect of cellular therapy for heart failure, we used a model of cardiac tissue to help us answer some of these fundamental questions in a controlled, in vitro quantitative setting.
Self-organized cardiac tissue (SOCT) uses the tendency of individual neonatal rat cardiomyocytes to aggregate into three-dimensional constructs that have the architectural properties of native cardiac tissue [7]. Importantly, this in vitro model of SOCT replicates cardiac function with spontaneous contractions. The force generated by the contraction of SOCT can be measured with a sensitive force transducer. Pharmacologic agents such as thyroxine and Clenbuterol have been shown to augment force production of SOCT [8, 9]. In this study, we examined the effects of thymus-derived MSCs on SOCT.
Material and Methods
The University of Michigan Institutional Animal Care and Use Committee approved the experimental protocol, and all animals were treated in compliance with the Guide for the Care and Use of Laboratory Animals of the National Academy of Sciences, published by the National Institutes of Health, revised 1996. Regarding human subjects, this study was performed in accordance with an accepted protocol from the University of Michigan Institutional Review Board. Parental consent was obtained to collect discarded thymus tissue.
Subjects
Discarded thymus tissue from infants (age < 2 years) with congenital heart disease undergoing cardiac surgery was used in this study. The thymus gland tissue was stored in a sterile container with buffer solution and transported to the laboratory within 6 hours for MSC isolation.
Mesenchymal Stromal Cell Isolation
Thymus gland tissue was fragmented, treated with 0.32 mg/mL type II collagenase solution for 90 minutes, and filtered through cell strainers to remove the stromal capsule. The cell suspension was centrifuged and suspended in Dulbecco's modified Eagle medium, with high-glucose concentration, GLUTAMAX I, 10% heat-inactivated adult bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Gibco, Carlsbad, CA) and then cultured at 37°C in a 5% CO2 humidified atmosphere. After 72 to 96 hours of culture, nonadherent cells were removed, and adherent cells were washed and incubated until confluence in Dulbecco's modified Eagle medium growth medium. Cell culture medium was then changed twice a week. When 70% to 80% adherent cells were confluent, they were split (0.25% trypsin at 37°C for 5 minutes; Gibco) and expanded.
Multilineage Potential of Thymus-Derived Mesenchymal Stromal Cells
Differentiation media was used to induce adipocytic, chondrogenic, and osteogenic differentiation using a commercially available kit (StemPro Differentiation Kits, Invitrogen, Carlsbad, CA); resulting cell populations were subsequently stained for identification according to the manufacturer's instructions.
Isolation of Neonatal Rat Cardiomyocytes
Neonatal rat cardiomyocytes were isolated from 2- to 3-day-old F344 rat hearts as described [8]. Briefly, hearts were minced and suspended in a dissociation solution (0.32 mg/mL collagenase type II and 0.6 mg/mL pancreatin dissolved in a buffer consisting of 116 mmol/L NaCl, 20 mmol/L HEPES, 1 mmol/L Na2HPO4, 5.5 mmol/L glucose, 5.4 mmol/L KCl, and 0.8 mmol/L MgSO4). Enzymatic dissociation was carried out in an orbital shaker for 30 minutes at 37°C, media was replaced, and dissociation on an orbital shaker was continued for another 30 minutes. Cells from all digests were pooled, centrifuged, and then suspended in plating medium consisting of 64% (vol/vol) M199, 20% F12K, 7% fetal bovine serum, 7% calf bovine serum, antibiotic–antimycotic, hydrocortisone 40 ng/mL, and ascorbic acid 40 μg/mL (Invitrogen) to achieve a final concentration of 1 × 106 cells/mL.
Preparation of Self-Organized Cardiac Tissue Plates and Fibers
Self-organized cardiac tissue fibers were created from self-organizing cardiomyocytes seeded on fibrin gel in modified 35-mm culture dishes that were coated with polydimethylsiloxane elastomer (Dow Chemical Corporation, Pittsburg, CA). Six-millimeter-long segments of 0-0 silk sutures (Ethicon, Somerville, NJ) were pinned 12 mm apart in the center of the culture plate and served as anchors for the SOCT fiber. Thrombin (10 U/mL) and fibrinogen (20 mg/mL) were added on top of the polydimethylsiloxane layer to form the fibrin gel layer. Neonatal rat cardiomyocytes (1 × 106 cells) were added to each plate after fibrin-gel formation. The cells were cultured under standard conditions with media changes every other day. Control groups contained only cardiomyocytes. For experimental groups, varying numbers of human thymus MSCs (1 × 105 to 1 × 106 cells) were added along with the neonatal rat cardiomyocytes to the culture dish at the time of plating. There were at least five SOCT fibers per group, and experiments were repeated with MSCs isolated from thymus gland tissue from three different patients. Self-organized cardiac tissue fibers were then kept at growth conditions until force testing was done at 14 days. Self-organization was measured by taking digital pictures of the forming fiber at daily intervals. Image-processing software (Adobe Photoshop; Adobe Systems Inc, San Jose, CA) was used to calculate the area circumscribed by the fibrin gel. Self-organization was calculated as 100 × [1 – (Areafibrin gel – Areaplate)/Areaplate], where Areaplate is the total area of the bottom of the 35-mm culture dish.
Thymus Mesenchymal Stromal Cell Green Fluorescent Protein Labeling
To gain insight into the distribution and fate of thymus MSCs within the SOCT, MSCs were labeled with green fluorescent protein (GFP). Thymus MSCs from the first passage were plated onto 12-well tissue culture plates suitable for direct microscopy (Becton Dickinson, Franklin Lakes, NJ). Mesenchymal stromal cells were allowed to recover and attach for approximately 24 to 36 hours, after which time the media was aspirated and replaced with Dulbecco's modified Eagle medium containing a GFP-expressing lentivirus prepared at the University of Michigan Vector Core facility. The plates were incubated overnight at 4°C, and then additional complete media with 20% fetal bovine serum was added to each well. Mesenchymal stromal cells were placed at 37°C for 24 hours. Media was then aspirated and replaced with fresh media. Expression of GFP was assayed using live fluorescent microscopy on a Zeiss Axioskop 2 Microscope running Axiovison software (Carl Zeiss, Inc, Jena, Germany) and was confirmed to be expressed in all cells. Medium was changed 24 hours after infection, and cultures were continued for downstream applications as described.
Self-Organized Cardiac Tissue Immunohistochemistry
Self-organized cardiac tissue fibers were constructed as described above with GFP-labeled thymus MSCs (no older than three passages), allowed to self-organize, and maintained in culture for 3 weeks. Self-organized cardiac tissue fibers were then fixed in 4% formalin and were paraffin-embedded and sectioned. Heat-induced epitope retrieval was done in 10 mmol/L citric acid buffer, pH 6.0, for 30 minutes, and sections were stained with antibodies to α-sarcomeric actin (mouse monoclonal) and GFP (rabbit polyclonal). Stained calls were visualized with Alexa488 and Alexa594 secondary antibodies (all antibodies were from Invitrogen), and nuclei were stained by mounting with 4=,6-diamidino-2-phenylindole–containing mounting media (Invitrogen). Appropriate isotype controls and control fibers without MSCs were stained in parallel. Slides were analyzed on a Zeiss Axioskop 2 Microscope running Axiovison software (Carl Zeiss, Inc).
Evaluation of Self-Organized Cardiac Tissue Fiber Force Generation
The force of spontaneously beating SOCT fibers was measured using a custom-built optical force transducer, and data were digitally recorded using LabView (National Instruments Corporation, Austin, TX). Self-organized cardiac tissue fibers in plating medium were maintained at 37°C on a heating plate as one end of the fiber was attached to the force-transducer load element and unpinned from the polydimethylsiloxane coating. Data were recorded using LabView at a rate of 1,000 points/s for a 10-second interval. The peak force (Fmax) and spontaneous contraction frequency were determined from the individual force tracings.
Self-Organized Cardiac Tissue Fiber Work and Power Output
Because the force-displacement (F – δ) relationship of the transducer is linear, the compliance is a measurable constant α = δ/F (3.4 mm/N). Because measurements are made in time (t), the mechanical work is therefore W(t) = αF(t)2/2, and the instantaneous power is P(t) = dW/dt = αF(t) dF(t)/dt. The force history, F(t), measured at 1-millisecond intervals, was smoothed and then numerically differentiated in time to give a power history, P(t). Both of these can be normalized by the specimen's reference volume, V0 = A0 L0, where A0 is area and L0 is length, to give work and power densities, metrics that are geometry independent.
Determination of Time Relaxation Constant
To investigate the effects of thymus MSCs on the diastolic function of SOCT fibers, the force decay at the time of maximal –dF/dt was fitted to the model of force (F) with respect to time (t):
where F∞ is the baseline force at diastole, F0 is the force at the maximal –dF/dt, and τ is the time relaxation constant. The above model of force decay has been widely used for pressure decay in the heart as an index of diastolic performance [10]. Mathematica 7 software (Wolfram Research, Champaign, IL) was used to perform the curve fitting and determination of τ for SOCT fibers. Force decay curves from at least three different SOCT fibers from each experimental group were analyzed, and the average τ was calculated.
Statistics
Averages and standard deviations of SOCT fiber peak force, contraction frequency, and τ were calculated for each group. The two-tailed Student's t test was used to evaluate differences in peak force, contraction frequency, and τ of SOCT fibers resulting from MSC addition. Microsoft Excel XP Professional Edition (Microsoft Corporation, Redmond, WA) was used for statistical analysis.
Results
Multidifferentiation Potency of Mesenchymal Stromal Cells
Plastic adherent cells were readily isolated from collagenase-digested neonatal human thymus glands. These cells exhibited the ability to acquire osteoblastic, chondrocytic, and adipocytic characteristics after incubation with specified differentiation media as expected for MSCs (data not shown).
Rate of Fiber Formation
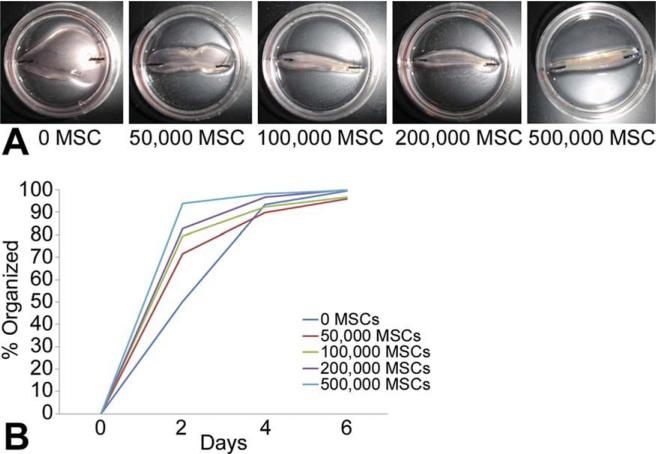
The formation of SOCT fibers had an initial rapid phase (approximately 24 hours) during which cells compacted about 25% of the fibrin gel as they self-organized (Fig 1). Self-organization then transitioned to a later, slower phase (72 to 96 hours) in which the cardiac tissue could be seen forming a distinct, well-aligned fiber. The effect of thymus MSCs on SOCT fiber formation was immediately apparent in the initial rapid phase. Self-organized cardiac tissue fibers containing thymus MSCs had a more rapid initial phase of self-organization, and this rate was dependent on the number of MSCs added. Thymus MSCs alone could form self-organized tissue fibers within 1 day (data not shown).
Fig 1.
Thymus mesenchymal stromal cells (MSCs) increase the rate of self-organization of cardiomyocytes. Thymus MSCs were added in increasing concentrations to 1 × 106 cardiomyocytes and allowed to self-organize in a custom culture dish. (A) Day 2 after plating, fibers containing more MSCs were more self-organized as compared with controls without MSCs. (B) Graphic representation of self-organization as a function of time and number of MSCs. Fibers with more MSCs self-organized more rapidly (n = 3/group).
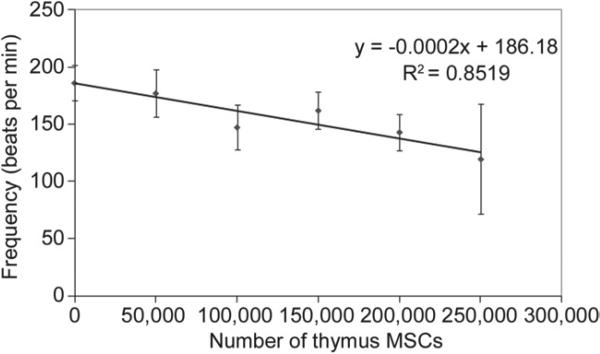
Contraction Frequency
Spontaneous contractions could be seen in all constructs under a low-power microscope (4×) within 48 hours of plating. The addition of thymus MSCs also decreased the spontaneous contraction frequency of SOCT fibers (Fig 2). This decrease in contraction frequency was directly related to the number of MSCs added, with the addition of 250,000 MSCs resulting in a 20% decrease in contraction frequency as compared with SOCT fibers without MSCs (p < 0.001).
Fig 2.
Thymus mesenchymal stromal cells (MSCs) decrease the rate of self-organized cardiac tissue fiber contraction frequency. Contraction frequency of self-organized cardiac tissue fibers containing thymus mesenchymal stromal cells were determined from force tracings. Increasing the amount of mesenchymal stromal cells in self-organized cardiac tissue fibers led to a decrease in self-organized cardiac tissue fiber contraction frequency, which followed an indirect relationship (n = 5/group).
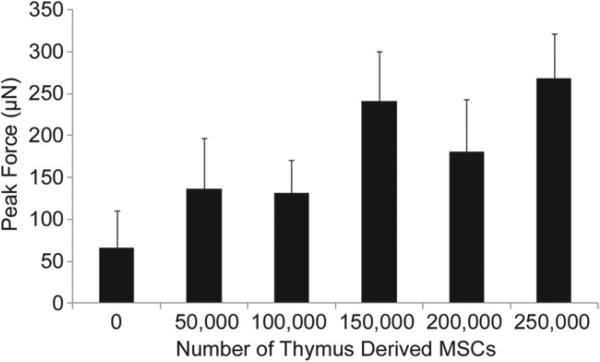
Force of Contraction
The addition of thymus MSCs resulted in a significant increase in SOCT fiber maximal force production. The magnitude of systolic enhancement ranged from twofold to fourfold as compared with the control SOCT fibers (Fig 3). However, the addition of larger numbers of MSCs resulted in a decrement in force production with 500,000 MSCs abolishing all contractile function (data not shown).
Fig 3.
Thymus mesenchymal stromal cells (MSCs) increase the force production of self-organized cardiac tissue fibers. Self-organized cardiac tissue fiber force production was measured by an optical force transducer. The addition of thymus mesenchymal stromal cells significantly augmented force production at all numbers shown here (n = 5/group; p < 0.05). The addition of 500,000 mesenchymal stromal cells abrogated all force production.
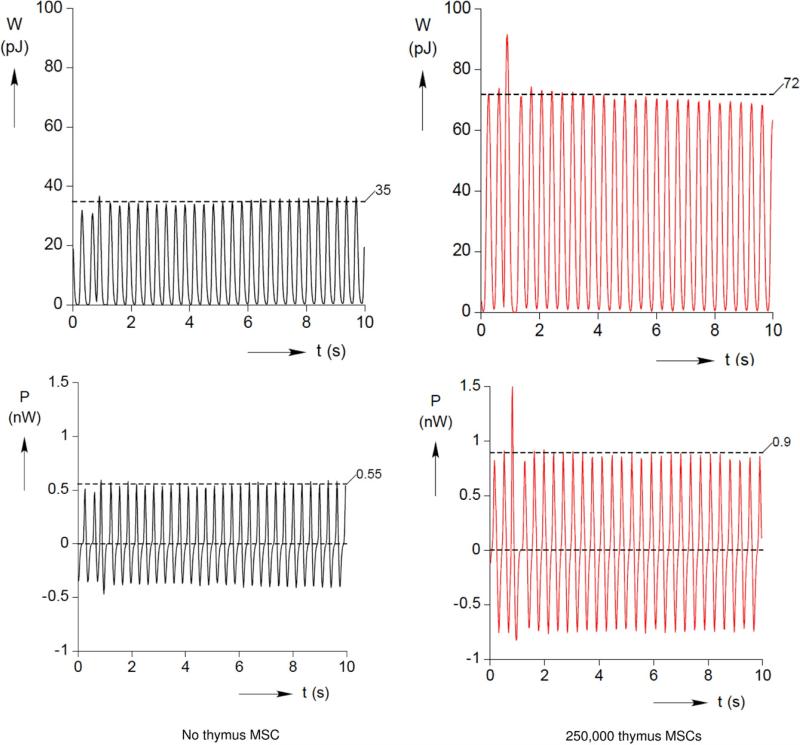
Work and Power
To determine the global effects of thymus MSCs on SOCT performance, SOCT fiber work and power production were numerically derived from the force measurement history F(t). In the best-case scenarios, instantaneous work and power were approximately doubled by the addition of MSCs (Fig 4).
Fig 4.
Thymus mesenchymal stromal cells (MSCs) increase work and power output of self-organized cardiac tissue fibers. Work and power outputs were numerically derived from the measured force history, F(t). Typical work and power output curves are shown here for a control self-organized cardiac tissue fiber and one containing 250,000 mesenchymal stromal cells.
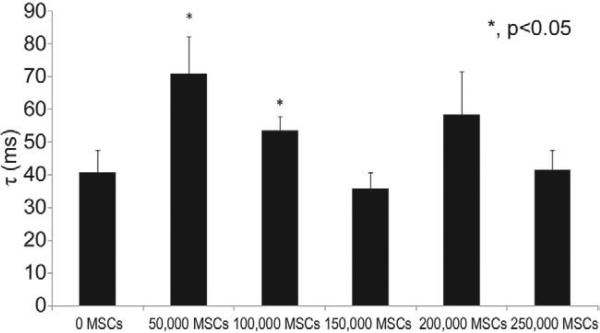
Time Relaxation Constant (Diastolic Function)
Force decay curves for SOCT fiber with and without thymus MSCs were analyzed in an identical manner to that used for the study of the pressure decay curves in intact hearts to estimate diastolic function [10]. The lower numbers of MSCs evaluated significantly increased the time relaxation constant of the SOCT fibers as compared with the control group, indicating a decrement in diastolic function (Fig 5).
Fig 5.
Thymus mesenchymal stromal cells (MSCs) improve diastolic function of self-organized cardiac tissue fibers. The time relaxation constant (τ) was calculated from the force history, F(t), of self-organized cardiac tissue fibers. Lower numbers of mesenchymal stromal cells evaluated decreased the time relaxation constant as indicated (n = 4 – 6/group).
Immunohistochemistry
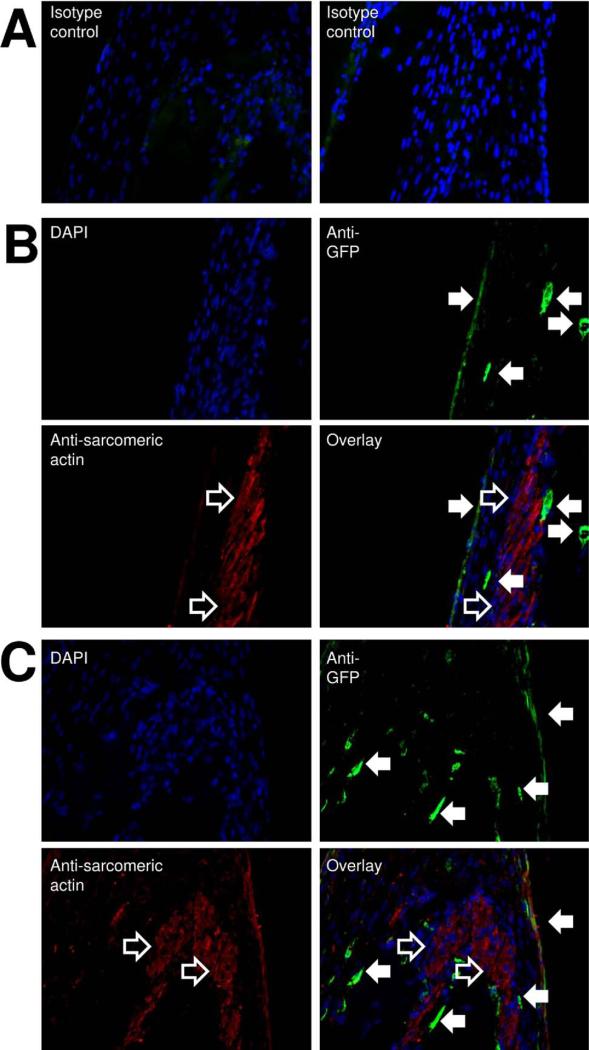
To investigate the fate of thymus MSCs in SOCT, MSCs were transduced with a lentivirus carrying the GFP gene and allowed to self-organize with neonatal rat cardiomyocytes into SOCT. Immunohistochemical studies with minimal isotype antibody signal (Fig 6A) revealed that cardiomyocytes were not oriented parallel to the axis of the SOCT fiber: they attained a spiral configuration (Fig 6B). This arrangement explains the twisting motion observed during SOCT fiber contraction (data not shown). Islands of fibrin gel formed the core of the SOCT fiber.
Fig 6.
Self-organized cardiac tissue fiber ordered structure. Thymus mesenchymal stromal cells (MSCs) with green fluorescent protein (GFP) and cardiomyocytes self-organize into fiber with a defined pattern. (A) Isotype antibody staining reveals minimal background signal. (B) In some sections, tightly packed cardiomyocytes (open arrows) attain a configuration at an angle to the long axis (spiral configuration). (C) Fibrin gel forms the core of the fiber that is surrounded by green fluorescent protein–positive mesenchymal stromal cells (anti-green fluorescent protein, green, closed arrows). Cardiomyocytes (anti-sarcomeric actin, red) are organized around the fiber core. A stratified layer of cells with weak anti-sarcomeric and anti-green fluorescent protein signals forms the outer layer of the self-organized cardiac tissue fiber. All fibers shown here (100×) were created from 100,000 mesenchymal stromal cells and 1 × 106 cardiomyocytes.
Thymus MSCs transduced with GFP were not distributed randomly or homogeneously throughout the SOCT fiber. They attained a location either on the outside of the SOCT fiber or bordering the cardiomyocytes internally at the periphery of the islands of fibrin gel. These cells had a strong GFP signal and did not colocalize with cardiac sarcomeric actin (Fig 6C).
Sarcomeric actin stained intensely in the cardiomyocytes as expected. The periphery of the SOCT fiber with MSCs stained weakly with sarcomeric actin and GFP. Cells in the periphery of the SOCT fiber were stratified and had a positive but weak signal for GFP. This suggests possible proliferation and differentiation of MSCs externally. No differences were noted between fibers containing different ratios of MSCs to cardiomyocytes in terms of the distribution and pattern of either GFP or sarcomeric actin staining.
Comment
This study demonstrates the utility of thymus MSCs as a therapeutic agent. The presence, isolation, and culture of human thymus MSCs have been described previously [11–15]. Recently, the isolation of thymus MSCs from neonates undergoing cardiac surgery has been described [12, 15]. These two groups of investigators isolated thymus MSCs using collagenase digestion similar to our method and performed a detailed phenotypic characterization. They found that thymus MSCs were CD73 positive, CD105 positive, and CD45 negative. Mouiseddine and associates [12] also found that 30% of CD73-positive and CD105-positive cells were also CD34 positive but lacked CD11b expression. Both groups of investigators demonstrated the multilineage potential of thymus MSCs into adipocytic, chondrocytic, and osteoblastic lineages as we had done here. Both groups demonstrated that thymus MSCs inhibited the mixed lymphocyte reaction, another established property of MSCs.
Thymus MSCs at least doubled the amount of force generated by SOCT at ideal ratios to the number of cardiomyocytes. Thymus MSCs also improved the global performance of SOCT as demonstrated by improved work and power output. Although it is quite evident that the systolic performance of SOCT is improved by thymus MSCs, the diastolic performance of SOCT is actually improved by the lower numbers of MSCs evaluated. The decrease in diastolic performance could be explained by an increase in the stiffness of the SOCT by the MSCs. The increase in stiffness may be secondary to the MSCs themselves or the increase in extracellular matrix produced by the MSCs. However, with this postulate, we would expect that increasing the number of MSCs would progressively make the SOCT fiber stiffer and decrease diastolic performance, which was not the case. It could be that there is an optimal ratio of MSCs for increasing diastolic and systolic performance, and further studies would need to confirm this hypothesis. These results also suggest that too many MSCs can actually be detrimental to cardiac function, and this is relevant to the current clinical approaches of injecting large amounts of MSCs directly into the myocardium, which may result in a high local MSC to cardiomyocyte ratio and thus decreased cardiac muscle function.
The improvement in SOCT fiber systolic performance was significant and marked. The ratio of neonatal rat cardiomyocyte to human thymus MSC yielding the optimal systolic and diastolic function was approximately 1:5. The improvement in systolic function could be attributable to (1) extracellular matrix produced by MSCs, (2) paracrine effects, (3) cardiomyocyte arrangement in the SOCT fiber, (4) transdifferentiation of MSCs into contractile cells, and (5) transfer of mitochondria from MSCs to cardiomyocytes.
Transfer of mitochondria has previously been show to occur between MSCs and somatic cells with defective mitochondria [16]. Although we were not able to trace the origin of the cardiomyocyte mitochondria in our SOCT fiber, it is an attractive theory to explain the observed functional improvement in the present study. The immunohistochemistry results suggest that MSCs do not transdifferentiate into cardiomyocytes. The cardiomyocytes were uniformly negative for GFP, and although we cannot rule out rare events of cell fusion or transdifferentiation of donor MSCs, this does not appear to be the major mechanism responsible for the observed increase in force generation. Furthermore, the decrease in spontaneous contraction frequency with incremental addition of thymus MSCs suggests that these cells are acting as current sinks to the SOCT in a mechanism that is identical to the decrease in conduction velocity in cardiac tissue caused by fibroblasts [17]. Although we were unable to visualize the exact mechanism by which thymus MSCs improved SOCT fiber formation at high resolution, it was evident that addition of MSCs improved fiber formation beyond direct differentiation into effector cells. We speculate that the main mechanisms responsible for the observed improvement are a combination of paracrine signaling and secretion of beneficial extracellular matrix.
Mesenchymal stromal cells alone were able to self-organize into tissue fibers much more rapidly than cardiomyocytes alone. Mixing MSCs and cardiomyocytes resulted in an increased rate of SOCT fiber formation, and this was directly related to the number of MSCs present. This rapid self-organization may help explain the beneficial effects of MSCs in wound healing of tissue defects. It is attractive to postulate that MSCs organized into a fiber first, which then served as a scaffold for the cardiomyocytes; however, the results of the immunohistochemistry demonstrate that in addition to the primary self-organization of these cells into fibers, there was a secondary self-organization of MSCs within the fiber into discrete locations within the fiber. Mesenchymal stromal cells were not primarily located at the core of the SOCT fiber and were mostly located at the periphery of the fiber. Mesenchymal stromal cells and neonatal rat cardiomyocytes were segregated within the SOCT fiber. Mesenchymal stromal cells were not dispersed within the neonatal rat cardiomyocytes.
Our work revalidates previous studies indicating a possible healing effect of MSCs on cardiac tissue. Using thymus MSCs in an environment that encourages cardiomyocytes to self-organize allowed us to study the effects of MSCs in conditions that more closely mimicked in vivo tissue than previous two-dimensional culture studies. Further work is being done to determine the mechanisms by which thymus MSCs improve the functional performance of SOCT. Although the present study is not intended to describe a novel method for in vitro cardiac tissue engineering, it is conceivable that a similar methodology may be used to generate transplantable cardiac tissue in the future. Any such clinical application of the SOCT described in the present study will, however, require extensive further evaluation of the underlying molecular and mechanical mechanisms. The model described herein will serve as an excellent platform for such future studies, and the knowledge obtained will hopefully lead to therapeutic strategies for heart failure.
DISCUSSION.
DR RICHARD A. HOPKINS (Kansas City, MO): I hope the audience recognizes what an important study and what an important avenue of study this represents. These are the kind of studies that really need to be done before we begin blindly injecting stem cells into muscle, and I really applaud you for your area of research.
I have a couple of questions. One, in your abstract you talk about incubation and differentiation media, the MSCs (mesenchymal stromal cells) attained the expected phenotype, what did you mean by that? Did you mean by that just the definition of the MSC as osteogenic, chondrogenic, and adipogenic-capable?
DR SI: That's correct.
DR HOPKINS: So you're not identifying a specific subpopulation of MSCs?
DR SI: No.
DR HOPKINS: And then secondly, MSCs contain alpha smooth muscle actin, and are, in fact, intensely contractile, as you know, as part of their reason for being. They migrate around and become other cells. So rather than a paracrine effect on the myocardiocytes, couldn't this be a codependent interaction of two different contractile cell types that give you the additional work that you're measuring with your force transducer? Let me commend an outstanding area of research.
DR SI: Thank you.
Well, you would expect, I mean it's certainly possible that these MSCs, as you pointed out, do contain contractile proteins, but I find it hard to believe that these cells should double or quadruple the amount of force generated by these tissue fibers. And these are added not in high concentrations, as I've pointed out, yet have beneficial effects when it's less than 1:5 ratio. So my feeling is that it's probably a paracrine effect. Also, we're looking into the extracellular matrix production by these cells and how they affect cardiomyocyte function.
DR FRANK SCHOLL (Hollywood, FL): That was an excellent and very elegant study and I congratulate you on this work. And my question actually relates to something you just said. As we learn more about stem cells, we're learning more that it may not be the cells themselves but the reaction and interaction with the extracellular matrix. And it appears that maybe in heart failure some of this extracellular matrix is damaged or different. Have you looked at, as you look forward, how this could become therapeutic, testing this hypothesis in an environment that may mimic a failing heart as opposed to the more steady state you have?
DR SI: Right. I mean this is just, as you point out, just an initial study into self-organized cardiac tissue. And as I pointed out earlier, to bring this a step forward to look at an injury model, we can certainly generate patches, we can certainly injure them, either by heat or cryo, and then attempt our therapies by injection, or whatever we think is optimal and look at the beneficial effects this way.
Because in this project I actually added the cells along at the time of self-organization, so that is not going to be the case you see clinically. But I think it's a good start, and it actually shows what the maximum potential you can get is in terms of beneficial effects from stem cell therapy in cardiac tissue regeneration. We have improvement in systolic function of up to three to four times. And certainly all of the clinical studies so far of a direct injection have at most only a few percent of improvement in systolic function.
Notice From the American Board of Thoracic Surgery.
The 2010 Part I (written) examination will be held on Monday, November 22, 2010. It is planned that the examination will be given at multiple sites throughout the United States using an electronic format. The closing date for registration was August 15, 2010. Those wishing to be considered for examination must apply online at www.abts.org.
To be admissible to the Part II (oral) examination, a candidate must have successfully completed the Part I (written) examination.
A candidate applying for admission to the certifying examination must fulfill all the requirements of the Board in force at the time the application is received.
Please address all communications to the American Board of Thoracic Surgery, 633 N St. Clair St, Suite 2320, Chicago, IL 60611; telephone: (312) 202-5900; fax: (312) 202-5960; info@abts.org.
Acknowledgments
This work was supported by the Section of Cardiac Surgery, University of Michigan, UC Davis School of Medicine, and an AMA Research Seed Grant awarded to Dr Ming-Sing Si.
Footnotes
Presented at the Forty-sixth Annual Meeting of The Society of Thoracic Surgeons, Fort Lauderdale, FL, Jan 25–27, 2010.
References
- 1.Rosamond W, Flegal K, Friday G, et al. Heart disease and stroke statistics—2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Norozi K, Wessel A, Alpers V, et al. Incidence and risk distribution of heart failure in adolescents and adults with congenital heart disease after cardiac surgery. Am J Cardiol. 2006;97:1238–43. doi: 10.1016/j.amjcard.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia R, Hare JM. Mesenchymal stem cells: future source for reparative medicine. Congest Heart Fail. 2005;11:87–93. doi: 10.1111/j.1527-5299.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- 4.Zimmet JM, Hare JM. Emerging role for bone marrow derived mesenchymal stem cells in myocardial regenerative therapy. Basic Res Cardiol. 2005;100:471–81. doi: 10.1007/s00395-005-0553-4. [DOI] [PubMed] [Google Scholar]
- 5.Dawn B, Abdel-Latif A, Sanganalmath SK, Flaherty MP, Zuba-Surma EK. Cardiac repair with adult bone marrow-derived cells: the clinical evidence. Antioxid Redox Signal. 2009;11:1865–82. doi: 10.1089/ars.2009.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdel-Latif A, Bolli R, Tleyjeh IM, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 7.Baar K, Birla R, Boluyt MO, Borschel GH, Arruda EM, Dennis RG. Self-organization of rat cardiac cells into contractile 3-D cardiac tissue. FASEB J. 2005;19:275–7. doi: 10.1096/fj.04-2034fje. [DOI] [PubMed] [Google Scholar]
- 8.Huang YC, Khait L, Birla RK. Modulating the functional performance of bioengineered heart muscle using growth factor stimulation. Ann Biomed Eng. 2008;36:1372–82. doi: 10.1007/s10439-008-9517-9. [DOI] [PubMed] [Google Scholar]
- 9.Khait L, Birla RK. Effect of thyroid hormone on the contractility of self-organized heart muscle. In Vitro Cell Dev Biol Anim. 2008;44:204–13. doi: 10.1007/s11626-008-9094-0. [DOI] [PubMed] [Google Scholar]
- 10.Yellin EL, Hori M, Yoran C, Sonnenblick EH, Gabbay S, Frater RW. Left ventricular relaxation in the filling and nonfilling intact canine heart. Am J Physiol. 1986;250:H620–9. doi: 10.1152/ajpheart.1986.250.4.H620. [DOI] [PubMed] [Google Scholar]
- 11.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–13. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 12.Mouiseddine M, Mathieu N, Stefani J, Demarquay C, Bertho JM. Characterization and histological localization of multi-potent mesenchymal stromal cells in the human postnatal thymus. Stem Cells Dev. 2008;17:1165–74. doi: 10.1089/scd.2007.0252. [DOI] [PubMed] [Google Scholar]
- 13.Musina RA, Bekchanova ES, Belyavskii AV, Sukhikh GT. Differentiation potential of mesenchymal stem cells of different origin. Bull Exp Biol Med. 2006;141:147–51. doi: 10.1007/s10517-006-0115-2. [DOI] [PubMed] [Google Scholar]
- 14.Rzhaninova AA, Gornostaeva SN, Goldshtein DV. Isolation and phenotypical characterization of mesenchymal stem cells from human fetal thymus. Bull Exp Biol Med. 2005;139:134–40. doi: 10.1007/s10517-005-0231-4. [DOI] [PubMed] [Google Scholar]
- 15.Siepe M, Thomsen AR, Duerkopp N, et al. Human neonatal thymus-derived mesenchymal stromal cells: characterization, differentiation, and immunomodulatory properties. Tissue Eng Part A. 2009;15:1787–96. doi: 10.1089/ten.tea.2008.0356. [DOI] [PubMed] [Google Scholar]
- 16.Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci U S A. 2006;103:1283–8. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fahrenbach JP, Mejia-Alvarez R, Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol (Lond) 2007;585:565–78. doi: 10.1113/jphysiol.2007.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]