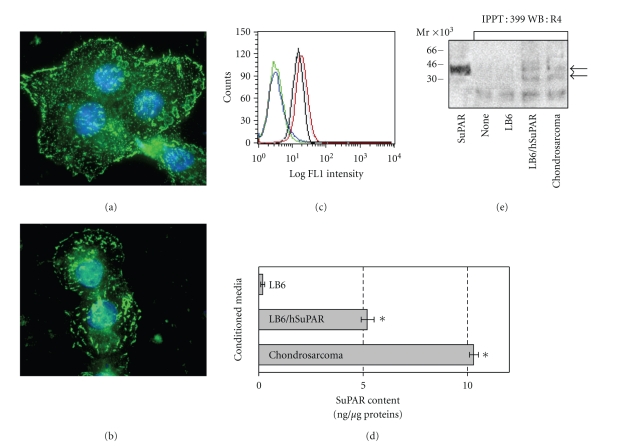
Figure 4.
Chondrosarcoma cells express uPAR and release its soluble forms in the medium. Chondrosarcoma cells were fixed with 2.5% formaldeyde (a) or fixed with 2.5% formaldeyde and permeabilized with 0.2% Triton X-100 (b) for 10 min at 4°C, incubated overnight at 4°C with 2 μg/mL R4 anti-uPAR mAb and then exposed to Alexa 488-conjugated F(ab')2 fragment of rabbit anti-mouse IgG (green). Nuclei were stained blue with DAPI. Original magnification: x1000. (c) Flow cytometry analysis of uPAR on chondrosarcoma and HT1080 cell surfaces. HT1080 and chondrosarcoma cells were harvested, incubated with normal rat serum added to PBS (green and blue curves, resp.) or 2 μg/mL R4 anti-uPAR mAb (black and red curves, resp.), stained with Alexa 488-conjugated F(ab')2 rabbit anti-mouse IgG and analyed by FACS. (d) Chondrosarcoma cells release soluble forms of uPAR in the medium. LB6, LB6/hSuPAR, or chondrosarcoma cells were grown to 80% confluence. Growth medium was removed, cells were extensively washed with PBS and then incubated in serum-free medium for 18 h. The medium was recovered, cleared by centrifugation, and analysed for SuPAR content by ELISA. Antigen concentrations were expressed as ng SuPAR per μg proteins. Columns, mean of two independent experiments; bars, ±SD, *P < .001 against the control (conditioned medium of LB6 wild-type cells). (e) 500 μL conditioned media of LB6, LB6/hSuPAR or chondrosarcoma cells were immunoprecipitated with 100 μL rabbit 399 anti-uPAR Ab conjugated to sepharose beads for 18 h at 4°C. The eluted proteins were separated by a 12.5% SDS-PAGE and analysed by Western blot using R4 anti-uPAR mAb. 1 μg purified SuPAR or 10 μL 399 anti-uPAR conjugated to sepharose beads (none) were loaded as a control. Arrows indicate the full-length SuPAR and the DIIDIII fragment of SuPAR.