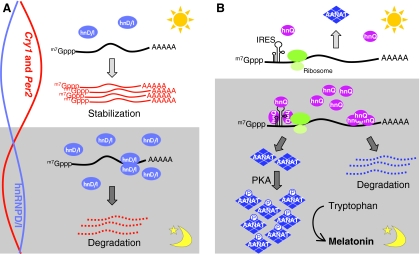
Fig. 3.
Post-transcriptional control of mammalian clock genes. (A) The regulation of Cry1 or Per2 mRNA stability by hnRNP D or I (hnD/I). During the night-time, the expression of hnRNP proteins in the cytoplasm is relatively high and more Cry1 and Per2 mRNAs are subject to degradation, leading to lower levels of mRNA (relative RNA levels depicted by curves on the left). By contrast, the level of hnRNPs is relatively low during the day; therefore, both Cry1 and Per2 mRNA can escape from the degradation caused by hnRNPs. Thus, these mRNAs become more stable, leading to higher mRNA levels. (B) Post-transcriptional and post-translational regulation of AANAT protein expression. During the night-time, hnRNP Q (hnQ) expression increases and this promotes the translation of AANAT through the interaction with IRESs. AANAT protein is phosphorylated by a PKA-mediated pathway and this prevents it from being degraded by the proteasome. Both of these post-transcriptional and post-translational regulatory steps contribute to the extreme amplitude of AANAT expression during the night. At the same time, hnRNP Q also binds to the 3′-UTR of Aanat mRNA and promotes degradation of Aanat mRNA. During the day, Aanat mRNA expression and the level of cytoplasmic hnRNP Q are low, and little or no AANAT protein is made. Melatonin is synthesized from tryptophan by four enzymatic steps, of which AANAT catalyzes the third to convert serotonin to N-acetylserotonin. The effects of hnRNP L and hnRNP R are not depicted here for simplicity. m7Gppp, 7-methylguanosine cap.