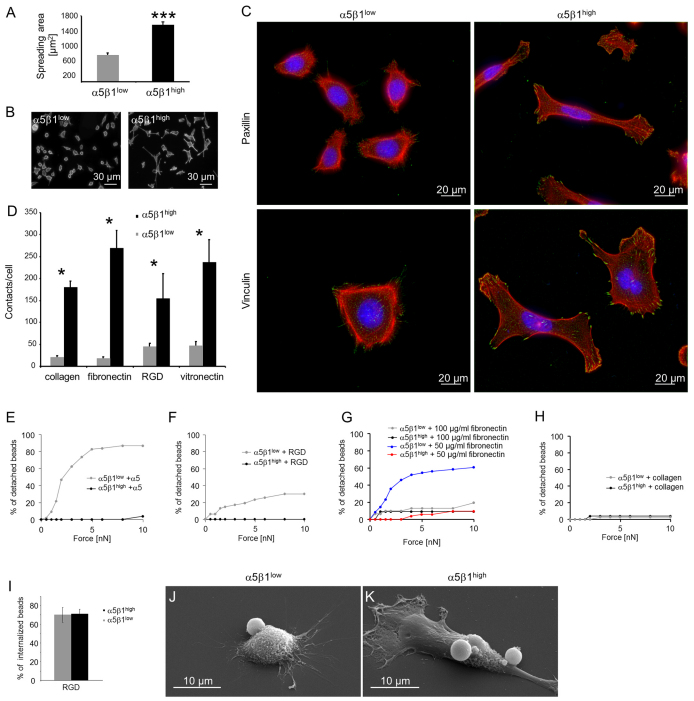
Fig. 6.
Adhesion of α5β1high and α5β1low cells. (A) Spreading area of α5β1high and α5β1low cells after 24 hours of adhesion to collagen-coated glass substrates. (B) Representative fluorescence images of α5β1high (right) and α5β1low (left) cells stained for actin with Alexa-Fluor-546-conjugated phalloidin. These images were used to determine the spreading area. (C) Fluorescence images of α5β1high (right) and α5β1low (left) cells stained for actin with Alexa-Fluor-546-conjugated phalloidin (red) and Hoechst 33342 for DNA (blue). Focal adhesions were stained with antibodies against vinculin (bottom) or paxillin (top) (green). (D) The number of adhesion contacts per cell (vinculin stained) was significantly increased in α5β1high as compared with α5β1low cells after 24 hours of adhesion on ECM proteins. (E,F) Increased bond stability (bead detachment) of integrin α5β1high cells towards anti-α5-antibody-coated (10 μg/ml antibody) beads (E) and 100 μg/ml RGD-peptide-coated beads (F) compared to α5β1low cells. (G,H) Bond stability of both subcell lines towards beads coated with100 μg/ml or 50 μg/ml fibronectin (G) and 100 μg/ml collagen (H). (I) Percentages of internalized RGD-peptide-coated beads after 30 minutes of incubation with α5β1high and α5β1low cells. (J,K) Representative SEM images of α5β1low (J) and α5β1high (K) cells that bound or internalized RGD peptide beads. *P<0.05, ***P<0.001.