Abstract
There is considerable interest in the role that mammalian heme peroxidase enzymes, primarily myeloperoxidase, eosinophil peroxidase and lactoperoxidase, may play in a wide range of human pathologies. This has been sparked by rapid developments in our understanding of the basic biochemistry of these enzymes, a greater understanding of the basic chemistry and biochemistry of the oxidants formed by these species, the development of biomarkers that can be used damage induced by these oxidants in vivo, and the recent identification of a number of compounds that show promise as inhibitors of these enzymes. Such compounds offer the possibility of modulating damage in a number of human pathologies. This reviews recent developments in our understanding of the biochemistry of myeloperoxidase, the oxidants that this enzyme generates, and the use of inhibitors to inhibit such damage.
Keywords: myeloperoxidase, hypochlorous acid, chloramines, protein oxidation, neutrophil
Overview of the Action of Myeloperoxidase and Other Heme Peroxidases
Activation of phagocytic leukocytes is a key process in the immune response to invading pathogens. Activation of these cells results in the assembly of a NADPH oxidase (NOX)-2 enzyme complex at the plasma membrane and a subsequent ’respiratory burst’, in which O2 is reduced, at the expense of NADPH, to superoxide radicals (O2•−).(1) This radical undergoes rapid spontaneous or catalysed (by superoxide dismutase) dismutation to give molecular oxygen and hydrogen peroxide (H2O2).(2) High concentrations of H2O2 can be bactericidal or cytotoxic, but lower levels have limited effects. Although this species can be reduced, by trace iron and copper ions, to hydroxyl radicals (HO•), the availability of these metal ions is usually very limited, and hence HO• is unlikely to be a major oxidant produced by activated neutrophils (reviewed in Ref. 3).
The bactericidal properties of activated leukocytes have been attributed, at least in part, to the actions of myeloperoxidase (MPO), a heme enzyme released by activated neutrophils, from intracellular granules. This green enzyme is the most abundant protein in neutrophils, accounting for up to 5% of their dry mass.(4) It is also present in monocytes, though at lower levels. The ability of monocytes to produce this protein decreases during maturation into macrophages, but evidence has been presented that macrophage-like cells, such as those detected in atherosclerotic lesions, have associated MPO.(5,6) The biological significance of MPO is evident from studies on people with total or incomplete MPO deficiency. Neutrophils from such people usually phagocytose foreign material normally, and have a prolonged respiratory burst that produces H2O2 and O2•− but MPO deficiency results in a greater risk of chronic infections.(7,8)
MPO protein has little bactericidal effect per se, but enzymatic reaction with H2O2 and halide (Cl−, Br−, I−) or pseudohalide (SCN−) ions generates hypohalous acids: hypochlorous acid (HOCl), hypobromous acid (HOBr), hypoiodous acid (HOI), and hypothiocyanous acid (HOSCN).(9–11) These oxidants are widely believed to be responsible for much of the anti-bactericidal activity of neutrophils, although other oxidants (including nitric oxide (NO•), peroxynitrite (ONOOH) and H2O2) and enzymatic systems (e.g., peptides, proteases, lysozyme) clearly also play an important role.
Although the generation of oxidants by MPO is beneficial in terms of the immune response to invading pathogens, there is considerable evidence that inappropriate stimulation of oxidant formation by this enzyme (wrong place, wrong time, excessive levels) can result in host tissue damage. Thus marked damage to cells and other biological materials (extracellular matrix, biological fluids) has been detected at sites of inflammation (reviewed in (10,12)). This damage has been linked with several human pathologies, and in at least some cases experimental and/or epidemiological evidence is available to suggest that oxidant generation by MPO (and/or other related heme peroxidases, such as eosinophil peroxidase (MPO)) is, at least partially, causal. This evidence for a role for MPO and its oxidants in human disease has been recently reviewed,(10,12) and will not be covered further here.
Physical Properties and Structure of Myeloperoxidase and Other Heme Peroxidases
Mature MPO is a cationic, dimeric, protein with a mass of 146 kDa, consisting of two 73 kDa monomers linked via a cystine bridge at Cys153. Each monomer, which is identical and functionally independent, consists of two components: a 58.5 kDa, 467 amino acid, heavy chain and 14.5 kDa, 106 amino acid, light chain.(13) The former is glycosylated and contains the modified iron protoporphyrin IX active site. The heme group sits at the bottom of a deep crevice,(14) which hinders access of most materials: only H2O2 and small anions have ready access to the iron atom.(15) Other materials that are oxidized by the enzyme (see below) bind in a hydrophobic pocket at the entrance to the distal heme cavity. The structures of human peroxidases have been recently reviewed (cf. Protein Data Base accession number 1cxp for MPO).(16)
Although MPO is the most widely studied heme-peroxidase, related species also play a critical role in metabolism, and some major human pathologies. EPO is the major granule protein of eosinophils, which are specialized human phagocytic cells that eliminate parasites and related organisms.(17) Unlike neutrophils, that phagocytose target organisms and subsequently release MPO into phagolysosomal compartments, eosinophils exocytose their granule contents on to the surface to which they are attached, as a result of the much larger size of most parasites. EPO shares a 70% amino acid homology with MPO and is also a cationic protein with a modified iron protoporphyrin IX heme active site.(18) It is synthesized as a ~80 kDa single-chain precursor and subsequently processed in to a mature protein (69.8 kDa) consisting of a heavy (57.9 kDa) chain and light chains (11.9 kDa); these are analogous of MPO.(16) Other related heme proteins include salivary peroxidase and lactoperoxidase (LPO); these are present in multiple human exocrine secretions including tears, milk, saliva and vaginal fluid. In each case their role appears to be as a defence against invading microorganisms.(19)
The genes for human MPO, EPO and LPO are adjacent to each other on chromosome 17 and have similar intron-exon structures, consistent with these arising via amplification from a common ancestor.(20) The cationic (pI ca. 10) nature of MPO and EPO (but not LPO) results in avid binding to negatively-charged structures including bacterial(21–23) and endothelial cell surfaces,(24) cytokeratin 1,(25) extracellular matrix components (especially polyanionic glycosaminoglycan chains(26–29)), albumin,(30) ceruloplasmin,(31) α1-antitrypsin,(32) and apolipoproteins A-I and B-100.(33,34)
Reactive Intermediates Generated by Heme Peroxidases
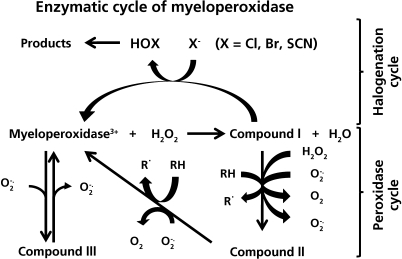
H2O2 reacts with the native, Fe3+ form of MPO to generate the two-electron oxidised species Compound I (an oxy-ferryl species, Fe4+ = O, with a porphyrin π-cation radical) and water. Compound I may be converted back to the ferric enzyme via two-electron reduction by (pseudo) halides (“the halogenation cycle”, Fig. 1), or via two sequential one-electron reduction reactions involving a second intermediate (Compound II, which retains the oxy-ferryl Fe4+ = O center; “the peroxidase cycle”, Fig. 1). An additional intermediate, Compound III, can be generated via reaction of the Fe3+ form with O2•−, or via one-electron reduction to the ferrous form and subsequent reaction with O2.
Fig. 1.
The enzymatic cycles of myeloperoxidase. Initial oxidation of the resting iron (III) form of the enzyme by hydrogen peroxide gives rise to Compound I, which is formally an iron (V) species. This intermediate can then undergo either two electron reduction with halide or pseudohalide ions to form hypohalous acids (the halogenation cycle) or undergo two successive one-electron reductions, via Compound II, with consequent radical formation (the peroxidase cycle). The iron (III) form of the enzyme can alos undergo one electron reduction with superoxide radicals to give Compound III. This latter reaction accounts for the SOD mimetic activity of myeloperoxidase.
Due to the high reduction potentials of the Compound I/native enzyme (1.16 V), Compound I/Compound II (1.35 V) and Compound II/native enzyme (0.97) couples, MPO can oxidize multiple substrates via its halogenation and peroxidase cycles. The unusually high potential for MPO arises from heme distortion and a reduction in electron density induced by a covalent vinyl sulfonium heme linkage.(35) These redox potentials, and hence the rate of substrate oxidation, are pH-dependent.(36) Compound III is unreactive towards most substrates, and is usually a catalytic ’dead-end’; this species will however slowly oxidize ascorbate(37) and paracetamol (acetaminophen)(38) to radicals, and is implicated in O2•−-dependent catalytic activities of MPO.(39)
The halogenation cycle
The ability of MPO to oxidize Cl− is unique amongst peroxidases; Br− and SCN− are also oxidized at high rates. Each of these substrates donates two electrons to Compound I to regenerate the ferric enzyme, with concomitant formation of the corresponding (pseudo) hypohalous acids (HOX, X = Cl, Br, SCN). The second order rate constants reflect the ease of oxidation of each substrate (SCN− > Br− > Cl−). However, the rate constants do not directly mirror the redox potentials as differences in topology of the active site, and binding sites exert a strong effect on substrate specificity.(16) Thus the rates of Br− and SCN− oxidation by EPO are ~10-fold faster than for MPO,(40) even though the MPO reduction potential is higher (1.10 vs 1.16 V). At neutral pH and physiological concentrations of halide/pseudohalide ions, MPO primarily generates HOCl and HOSCN(41) with the specificity constants for Cl−, Br− and SCN− being 1:60:730 respectively.(41) The yield of each hypohalous acid is donor dependent, as smoking and diet can markedly elevate SCN− levels and hence the extent of HOSCN formation from SCN−. Yields of 5–10% and 40%, based on H2O2 consumed, have been reported for HOBr and HOSCN formation by MPO, using mean physiological ion concentrations; HOCl accounts for most of the remainder.(41,42) In contrast, EPO primarily generates HOBr and HOSCN, and no HOCl;(43) LPO primarily generates HOSCN.(44) The yields of these materials is also modulated by secondary reactions; HOCl and HOBr can oxidize SCN−, and HOCl can oxidize Br−,(45,46) possibly via transhalogen species (e.g., BrCl), to the corresponding (pseudo) hypohalous acids.
The pKa values for of HOCl, HOBr and HOSCN are 7.59, 8.7 and 4.85–5.3, respectively.(47–49) Thus at physiological pHs, approximately equal concentration of HOCl and −OCl will be present, HOBr predominates over −OBr, and −OSCN predominates over HOSCN The physiological mixtures of these species are referred to as HOCl, HOBr and HOSCN respectively, from hereon. The rates of formation of these species by Compound I are significantly greater at acidic pH,(40) with this believed to be due to protonation of the distal histidine.(16,50)
Whilst previous studies have disputed the formation of “free” HOCl by MPO/H2O2/Cl−,(36,51) halogenation of large biological targets, which cannot enter the enzyme active site, occurs both rapidly and in near stoichiometric amounts (⩾75% for heparan sulfate based on H2O2 supplied(27,52)), consistent with the formation of diffusible oxidants.
The peroxidase cycle
In the peroxidase cycle, radicals are generated from substrates (both organic and inorganic) via one-electron oxidation by Compounds I and II. O2•− and NO• are also oxidized. The catalytic activity of MPO is therefore partitioned between halogenation and peroxidation via competition between peroxidase substrates and (pseudo) halides for Compound I; this partitioning is relevant to inhibition of enzymatic activity (see below).
Physiologically- and pathologically-relevant peroxidase substrates for MPO, EPO and LPO include endogenous species (e.g., the amino acids tyrosine and tryptophan, thiols, ascorbate, steroid hormones and urate) as well as xenobiotics and drugs. MPO oxidizes a wider range of substrates than EPO and LPO due to its higher reduction potential for the Compound I/Compound II couple (1.35 vs 1.14 V for LPO(53,54)). Substrates that react readily with Compound I, but not Compound II, i.e. species with reduction potentials between 1.35 and 0.97 V, are often termed ’poor’ peroxidase substrates. In the absence of additional species that can recycle Compound II, metabolism of such substrates results in Compound II accumulation and arrest of the catalytic cycle (see Inhibition section below). Kinetic factors can also affect recycling of Compound II and even with peroxidase substrates such as Tyr, the rate constant for reaction with MPO Compound II is ~10 times slower than for Compound I.(55)
The metabolism of Tyr by MPO and LPO is relatively insensitive to pH.(55,56) With MPO, rate constants for reaction of Tyr with Compound I are maximal at basic pH values, but vary less than two-fold across physiologically-relevant pHs.(55) The predominance of the chlorinating activity of MPO at acidic pH, over its peroxidative metabolism, can be accounted for primarily by the greater pH-dependence of Cl− oxidation.
Reactions of Hypohalous Acids
Hypochlorous acid
HOCl reacts rapidly with sulfur and nitrogen atoms (for rate constants see (57–59)), including those present in thiols, thioethers, amines, and amides. Thus, Cys residues in proteins and glutathione (GSH) are key targets.(57,60–63) Cys oxidation appears to yield a sulfenyl chloride (RS-Cl), which undergoes rapid reaction with excess thiol, to give the disulfide,(60) or with water to yield sulfenic (RSOH), sulfinic (RSO2H), and sulfonic (cysteic acid, RSO3H) acids [reviewed in (64)]. Disulfides (e.g., cystine) can be further oxidized to sulfonic acids via S-chlorinated and S-oxygenated intermediates [reviewed in (64)]. HOCl can also induce the formation of sulfenamide (RSNR'), sulfinamide [RS(O)NR'], and sulfonamide [RS(O)2NR'] cross-links in peptides (e.g., GSH) and proteins,(65–67) via nucleophilic attack of Lys or Arg side chains on RS-Cl, sulfenic or sulfinic acid intermediates. Glutathione sulfonamide (GSSG), formed from oxidation of GSH,(68) has been postulated as a potential marker for MPO-mediated damage in biological systems, as it is generated primarily by HOCl, and to a much lesser extent by other species such as HOBr and ONOO−/ONOOH.(66,69)
The high susceptibility of Cys residues to oxidation has important biological implications as this can disrupt the cellular redox balance of cells by conversion of GSH to GSSG, and inactivate multiple cellular enzymes that contain active site Cys residues. Thus, creatine kinase and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) are rapidly inactivated by low levels of HOCl, with this correlating with thiol depletion.(70) Conversely, HOCl can activate the (inactive) pro- forms of matrix metalloproteinases (MMP) (e.g., MMP-7) via conversion of a key Cys residue in the “cysteine switch” domain of pro-MMP-7 to a sulfinic acid.(71) Thioethers such as Met side chains are also favored targets, with this resulting in sulfoxide formation(72) (and sulfones with very high HOCl excesses(73)). These compounds are not useful as markers for HOCl damage, as they are also produced by other oxidants and can be repaired, intracellularly, by methionine sulfoxide reductases.(74) Met oxidation can lead to alterations in protein function by, for example, inactivating enzymes (e.g., lysozyme(72)), protease inhibitors (such as α1-antitrypsin),(72,75) proteinases,(76) growth factors (e.g., GroEl(77)) and modulating signaling pathways (e.g., oxidation of IkB with resulting inhibition of NF-kB activity(78)).
Amines, and to a lesser extent amides, are readily chlorinated by HOCl to give chloramines (RR'NCl) and chloramides (RC(O)N(R')Cl).(57,79,80) Dichlorination also occurs at high HOCl excesses. Chloramines are formed on multiple biological targets including the nitrogen atoms on: the α-amino and side-chains of His, Lys and Arg of free amino acids and proteins; taurine; free nucleobases, nucleosides, nucleotides and DNA/RNA; amino sugars and glycosaminoglycans (e.g., hyaluronan, heparan sulfates, heparin); and on the amine-containing head groups of phospholipids (e.g., phosphatidylethanolamine, phosphatidylserine).(58,59,64,81–85)
In addition to reaction with nucleophiles, HOCl undergoes addition to aromatic rings and double bonds, including some amino acids (Tyr and Trp), nucleobases, and fatty acid side chains [reviewed in (64,82)]. Addition to the phenolic ring of Tyr yields 3-chlorotyrosine (3-chloroTyr), which is widely used as a specific marker of HOCl generation.(86,87) Secondary halogenation to give 3,5-dichlorotyrosine can also occur. Oxidation of Trp residues is relatively rapid with this resulting in the formation of multiple products [reviewed in Ref. 10, 64], including cyclized species when the Trp residue is present in specific peptide sequences.(88)
Reaction of HOCl with nucleobases generates stable halogenated products, in addition to unstable chloramines, with these including 5-chlorocytosine, 5-chloro (2'-deoxy) cytidine, 5-chlorouracil, 8-chloroadenine, 8-chloro (2'-deoxy) adenosine, and 8-chloro (2'-deoxy) guanosine.(89–99) Some of these products have been utilized as markers of HOCl generation.(92–100) Hydroxylated and ring-opened species are also formed.(94,96)
HOCl reacts with the double bonds of unsaturated lipids and cholesterol to give chlorohydrins (RCH(Cl)-CH(OH)R'); these can subsequently yield epoxides.(36,101–107) Reaction with plasmalogen lipids, which contain a vinyl ether rather than an ester linkage, is particularly rapid(108) with this resulting in facile cleavage of the ether linkage to give an α-halogenated aldehyde and a lysophospholipid.(109,110) Elevated levels of the former have been detected in human atherosclerotic lesions.(111)
Hypobromous acid
HOBr is generally reactive and less discriminating than HOCl (for kinetic data see (58,112,113)), though many of the reactions are analogous to those of HOCl. It also targets thiol, thioether, disulfide and amine functions, with the last of these generating bromamines and bromamides. A major difference to HOCl is in the kinetics of reaction with aromatic rings and double bonds, with these being more rapid and important with HOBr.(58) Thus bromination of Tyr residues (to give 3-bromoTyr) is ~10,000-fold faster than chlorination by HOCl.(112) This has important ramifications for the use of 3-bromoTyr and 3-chloroTyr as markers of damage.(112) Similarly bromohydrin formation is more rapid than chlorohydrin generation by HOCl, and bromohydrins are more readily detected in biological systems.(114)
Hypothiocyanous acid
HOSCN has been postulated to be a significant product of MPO-mediated reactions, particularly in smokers.(115) Elevated levels of SCN− are present in such people as a result of hydrogen cyanide formation (from cigarette combustion) and subsequent metabolism. Non-smokers typically have plasma SCN− concentrations <50 µM, whereas heavy smokers have levels ⩽250 µM.(116,117) This is believed to result in increased formation of HOSCN over other MPO-derived oxidants (see above and (118)). Furthermore, reaction of HOCl and HOBr with SCN− can enhance HOSCN formation.
Unlike HOCl and HOBr, which are relatively promiscuous oxidants, HOSCN is less reactive and highly selective. Thiols are the major site of reaction,(49,119) though Trp residues are also oxidised when Cys residues are absent or depleted.(120) This observation is consistent with rapid reversible Cys oxidation, and low levels of irreversible incorporation of radiolabelled SCN− into proteins.(119–121) Thiol oxidation occurs via short-lived RS-SCN species which rapidly react with other thiols to give disulfides (and regenerate SCN−), or water to give a sulfenic acid (RS-OH). The cellular effects of this oxidant are therefore, not surprisingly, linked to damage to enzymes containing critical Cys residues, including GAPDH(63) and protein tyrosine phosphatases;(122) inhibition of the latter enzymes results in increased levels of cellular protein tyrosine phosphorylation (i.e. hyperphosphorylation) and altered mitogen-activated protein kinase (MAPK) signalling. These alterations are believed to underlie the enhanced apoptosis observed with this oxidant in some cells.(63,122)
There is little data available as to other direct reactions of HOSCN with biological targets, though it has been reported that a MPO/H2O2/SCN− system induces low-density lipoprotein (LDL) modification, with resulting formation of conjugated dienes and lipid hydroperoxides.(123) The detection of these products implies the occurrence of radical reactions, so this may arise from the peroxidation cycle of MPO rather than via HOSCN, though there is also evidence for the formation of radicals from MPO-catalysed oxidation of SCN−.(115)
Radicals
A number of different types of radical can be generated by the peroxidase cycle of MPO i.e. as a result of one-electron oxidation by Compounds I and II. As the redox potential of Compound I is greater than that of Compound II, the former can oxidise a wider range of materials. Some substrates can be oxidised by Compound I, and not (or poorly) by Compound II resulting in “trapping”, at least in vitro, of MPO at this point in its enzymatic cycle. Whether such “trapping” occurs in vivo is unclear at present, as multiple reductants (e.g., ascorbate, O2•−) that can convert Compound II back to the Fe3+ (native) form. Regardless of this, a greater availability of peroxidase substrates relative to halide ions is likely to diminish the yield of hypohalous acids (HOCl, HOBr and HOSCN) and result in higher radical yields. Whether this changes the overall extent of biological damage remains an open question, with this likely to depend, to a major extent, on the chemistry of the radicals formed (i.e. whether they are highly reactive and induce further damage, or are unreactive).
In some cases, termination reactions in the form of radical-radical dimerisation, appears to be a major fate. Thus dimers and higher polymers have been detected from phenols (e.g., dityrosine from Tyr oxidation) with the occurrence of these reactions minimizing further damage. Some radicals can also reduce native MPO to Fe2+ MPO, which generates Compound III upon reaction with O2. This occurs, for example, during MPO-mediated metabolism of hydroquinone,(124,125) amsacrine,(126) hydrazines(127) and hydrazides.(128) Other fates of MPO-generated radicals include reaction with the parent proteins to generate protein-derived radicals(129) and covalent addition to heme.(130,131) Radicals may also diffuse away from the MPO and damage other biomolecules including lipids(132,133) and proteins.(134) Radicals formed on oxidation of (amino) phenols can undergo further one-electron oxidation or disproportionation to generate electrophilic quinones/quinimines that form covalent adducts with thiols (e.g., GSH) and other biomolecules.(135,136) A number of drugs and xenobiotics induce adverse biological effects, including agranulocytosis, hepatotoxicity and cancer, which have been associated with their metabolism by heme peroxidases.(136,137)
Reactions of Secondary Oxidation Products
The damaging actions of MPO persist for considerable periods after the cessation of initial oxidant (e.g., HOCl) production.(79) Much of the secondary damage is believed to arise from the reaction of long-lived chloramines/chloramides and/or bromamines/bromamides, formed via the reaction of HOCl/HOBr with amines and amides (see above). The longer lifetimes of these species allow diffusion away from the site of formation (e.g., through cellular membranes) and the initiation of oxidative damage at remote locations; thus extracellularly generated species may exert intracellular effects, with the extent of cell penetration being dependent on the structure of the halogenated species.(138–141) Reactive aldehydes and radicals may also play a significant role in inducing secondary damage (see above and below).
Chloramines and bromamines
Chloramines (RNHCl) and bromamines (RNHBr), and the corresponding amide species [RC(O)NClR'; RC(O)NBrR'] retain the oxidizing equivalents of the parent HOCl/HOBr and can induce further reactions.(81,142,143) Some of these processes regenerate the parent amine (which may result in an underestimate of the extent of damage) as a result of halogen transfer (e.g., Ref. 144, 145) or radical reactions (e.g., Ref. 146, 147), whereas others result in conversion of the amine group (e.g., via hydrolysis, probably via an imine) to an aldehyde and ammonia.(64,148–150) Aldehyde formation from bromamines occurs more readily than from chloramines.(72,151,152) The resulting carbonyls can react with protein or lipid amine groups to generate Schiff base imines, which can ultimately yield advanced glycation end products (AGEs); the latter have been linked to vascular disease.(153)
Halogenated amines and amides can decompose to give nitrogen-centred radicals and subsequently carbon-centred radicals by rearrangement reactions; both may initiate further damage. Radical formation is promoted by low-valent redox-active metal ions (Fe2+, Cu+) and O2•−.(84,146,147,154,155) Halamines oxidize thiols and thioethers (e.g., Cys and Met, respectively) though at slower rates than HOCl and HOBr.(58,140,156) The lower reactivity of these species results in more selective damage, and a more limited range of products. Low pKa Cys residues are particularly susceptible to oxidation, with this resulting in selective inactivation of some enzymes.(141) Thiols are primarily converted to disulfides and sulfenic/sulfonic acids (and not sulfonamides as observed with GSH(66)). These processes can result in the induction of apoptosis and necrosis.(141,142,157)
Activation of phagocytes has been reported to result in ~15% conversion of the HOCl formed to chloramines,(79) whilst reaction of HOCl or an MPO system with Escherichia coli results in up to 50% of the HOCl being recovered as bacteria-derived chloramines;(158) some of these materials have lifetimes of many hours in buffer or complete media. Experiments with PMA-activated neutrophils indicate that these chloramines are formed on both low- and high-molecular mass materials (as determined by mass fractionation).(158) The former appears to be primarily taurine chloramine (which is consistent with the high concentration of this material in neutrophils), but this result may be distorted by the discovery of rapid chlorine transfer between amine groups,(139,144,145) and the greater stability of this chloramine over other species. Many of the chloramines have been reported to be hydrophilic,(158) but this conclusion may also be perturbed by the more rapid cellular penetration of hydrophobic species, and hence greater rate of loss of these materials via subsequent intracellular reactions, compared to those that remained external to the cells.
Hydroperoxides
Recent studies have shown that O2•− can react with some of the radicals generated by the peroxidase activity of MPO. Thus phenols can be oxidised to organic hydroperoxides via phenoxyl radicals that subsequently undergo radical-radical termination reactions with O2•−.(159,160) Such reactions occur with both the free amino acid tyrosine (and various derivatives) and Tyr residues present on peptides and proteins.(159–163) Some of the factors that control the hydroperoxide yields relative to other products (e.g., dimeric species such as di-tyrosine) have been elucidated.(160) Similar O2•−-dependent reactions appear to occur with other (long-lived) radicals generated by MPO, including those formed on urate, indoles and other related species (e.g., melatonin and serotonin(164,165)). The hydroperoxides formed by these reactions may undergo further reactions resulting in the oxidation of thiols (by two-electron oxidation reactions) and or radicals (as a result of one-electron reduction by trace transition metal ions). Such reactions have been characterised with a range of other amino acid-, peptide- and protein-derived hydroperoxides.(166–169) These reactions may contribute to the secondary damage induced at sites of inflammation and may rationalize the detection of hydroxylated products from MPO-catalysed reactions.
Reactive aldehydes
Chloramines and bromamines can decompose to yield aldehydes and ammonia with this reaction being particularly facile with species formed on the (N-terminal) amine groups of free amino acids and peptides.(150,170) Those formed on side-chain amines (e.g., the є-amino group of Lys side chains) decompose via this route to only a minor extent,(171) though this site, and others including phospholipid head groups, can be modified via further reactions of carbonyls formed at different sites, via Schiff-base formation.(149,172) Some of these materials, such as p-hydroxyphenylacetaldehye derived from the oxidation of free Tyr residues, have been detected at elevated levels in diseased tissues, such as atherosclerotic lesions.(172) Oxidation of other free amino acids, such as Ser and Thr, can generate highly reactive aldehydes, such as glycoaldehyde and 2-hydroxypropanol (and the highly reactive α, β-unsaturated material acrolein by dehydration of 2-hydroxypropanol), via chloramines.(171) These materials react rapidly with amine (e.g., Lys) and guanidine groups (e.g., Arg residues) to products similar to the AGEs detected in people with diabetes. Many of these materials are potent protein cross-linking agents due to their bifunctional nature. These compounds also form adducts with phospholipids and DNA bases.(173) Although reactive aldehyde formation occurs in both simple, and cellular, systems the quantitative significance of such reactions with free amino acids (present in plasma at ⩽ low millimolar levels(174)) may be limited due to the much higher concentrations of proteins in vivo which have similar reactive sites.(118,168)
Cyanate
Cyanate (OCN−) is a downstream product of SCN− oxidation by MPO, with this arising predominantly from HOSCN.(175) Cyanate reacts with amines under physiological conditions, with this resulting in carbamylation of N-terminal amine groups and the Lys side chain; the latter results in homocitrulline formation.(117) Plasma protein-bound homocitrulline concentrations have been reported to be an independent predictor of cardiovascular disease (CVD), future myocardial infarction, stroke, and death, suggesting a potential role for HOSCN and SCN−-derived products in atherosclerosis.(117)
Modulation of Myeloperoxidase Catalytic Activity by Other Oxidants
Both endogenous and exogenous compounds can modify the activity of MPO, and thereby modulate the nature and extent of oxidant formation and biological damage induced by this enzyme.
Hydrogen peroxide
Although H2O2 initiates the catalytic cycles (halogenation and peroxidase) of MPO, it can also inhibit MPO by acting as a competitive substrate for Compound I, by reacting with Compound II to generate (catalytically-inactive) Compound III, and by irreversibly inactivating the enzyme.(11,16) In the absence of other substrates (an unlikely scenario in vivo) MPO displays significant catalase activity, due to direct two-electron reduction of Compound I by H2O2 to the native enzyme and to a slower, competing process initiated via one-electron reduction of Compound I by H2O2 to Compound II;(176) the latter process has been proposed to occur via formation of the ferrous enzyme.(177)
Superoxide radical anion
O2•− undergoes rapid one-electron transfer reactions with Compounds I, II and III of MPO.(39) O2•− rapidly converts ferric MPO to Compound III [formally an Fe(II) species with a bound oxygen molecule] which can be subsequently recycled to the ferric form. MPO can therefore act as a superoxide dismutase, with these reactions modulating halogenation and peroxidase activity.(39) Computational modelling of reactions within the neutrophil phagosome, where MPO concentrations are high (ca. 1 mM), indicates that most of the O2•− produced by the NADPH complex is consumed via the superoxide dismutase activity of MPO, and that efficient recycling of Compound III by O2•− ensures that phagosomal HOCl production is not constrained.(178) Under other situations however (e.g., when the MPO concentrations and fluxes of H2O2 are low and turnover of the ferric enzyme is rate limiting), Compound III formation can inhibit HOCl production (reviewed in Ref. 16, 39).
At high H2O2 fluxes, where reaction of Compound I with H2O2 results in Compound II accumulation, O2•− can maintain MPO-mediated chlorination by recycling Compound II to the ferric enzyme.(16,39,179) This activity is also important in maintaining MPO activity during the oxidation of poor peroxidase substrates (see also below).(39,180,181)
Interaction of MPO with O2•− can promote catalytic activities that appear to be independent of the halogenation and peroxidase cycles. MPO has been shown to hydroxylate aromatic substrates, such as phenols(182) and salicylate,(183) and oxidize melatonin via O2•−-dependent processes.(164) These reactions may occur via reaction of ferric MPO with O2•− to form Compound III, and involve a reactive intermediate such as singlet oxygen (1O2).(164) Aromatic hydroxylation is also observed during the metabolism of the hydrazide derivative isoniazid by MPO where Compound III is generated.(127) As discussed above, it is now established that O2•− can react with some of the radicals generated by MPO, to yield hydroperoxides, and subsequent decomposition of these species may account for the formation of some of these hydroxylated products.
Nitric oxide
NO• reacts rapidly with Compounds I and II of MPO via one-electron transfer.(184) The initial product of these reactions, the nitrosonium ion (NO+), is a short-lived species that reacts with water to yield NO2− which may ultimately result in the formation of nitrosylated products.(185) The interaction of NO• with MPO may therefore alter the distribution of redox intermediates during steady state catalysis, and the balance between halogenation and peroxidase cycles.(186–188) Although the reactions of NO• with MPO Compounds I and II are facile, MPO-dependent consumption of NO• in human plasma at physiologically-relevant fluxes (steady state << 1 µM) is likely to predominantly occur via reaction with radicals generated by the peroxidase cycle (e.g., tyrosyl and ascorbate radicals).(189)
Nitrite
NO2− is a major decomposition product of NO• and is generated by autoxidation (via the formation of N2O3) or from metabolism by heme proteins such as hemoglobin(190) or peroxidases (see above). NO2− reacts with Compound I and Compound II of MPO(191,192) to generate NO2•. With MPO a small but significant fraction of NO2− is oxidized to a species that can induce hydroxylation as well as nitration, a property shared by peroxynitrite (ONOO−/ONOOH). In contrast to free peroxynitrite, MPO-H2O2-NO2− induces aromatic hydroxylation only at acidic pH, and CO2 does not enhance aromatic nitration, consistent with an enzyme-bound intermediate.(193) Analogous experiments with LPO indicate that this intermediate may be a complex of ONOO− with MPO.(194)
Peroxynitrite
Whilst NO• and O2•− have important, independent interactions with MPO the radical-radical termination product of these species, ONOOH also reacts. Thus ferric MPO can promote aromatic nitration by peroxynitrite.(195) Ferric MPO appears to be directly converted to Compound II, consistent with the generation of NO2•.(196,197) Reaction with ferric MPO is faster at acidic pHs, with the pH dependency consistent with the pKa of ONOOH/ONOO−, suggesting that ONOOH is the reactant,(196) and that Compound II formation occurs via dissociation of an intermediate oxygen-coordinated complex of ONOO− (see also preceding section).(197) Peroxynitrite also rapidly converts Compound I and Compound III, to Compound II. Compound II does not appear to oxidize peroxynitrite, despite this process being thermodynamically favorable.(197)
Inhibition of Myeloperoxidase Activity
In the light of the data supporting a role for MPO in a range of human diseases (reviewed in (10,12)), there is considerable interest in the development of MPO inhibitors. A number of different strategies to limit oxidant formation and damage have been investigated; these are summarised below.
Limiting the availability of substrates for oxidant production
MPO-mediated oxidant generation can obviously be limited by decreasing the availability of H2O2. This can be achieved by inhibiting the membrane-bound NOX of phagocytes, and related NOXs present in other cells (e.g., endothelial and smooth muscle cells(198,199)). A number of such compounds have been identified,(200,201) with the most extensively studied being diphenylene iodonium chloride (DPI).(202,203) This compound also inhibits other sources of O2•− formation (and hence H2O2 levels) including mitochondrial activities.(204) Despite the undoubted utility of these compounds in isolated systems, the plethora of sources of O2•− and H2O2 in vivo, may limit the usefulness of this strategy in some cases. Furthermore, low levels of H2O2 appear to be required for cell signalling, and hence limiting O2•− formation may not be always beneficial.
NO• can suppress NOX activity in endothelial cells via S-nitrosylation of the p47phox subunit(205) and thus may limit MPO-mediated damage by decreasing vascular H2O2 concentrations, in addition to its direct effects on MPO activity (see above). Whether sufficient NO• is present in vivo to have a major modulatory effect on MPO activity, in addition to its multitude of other effects, remains an open question. Oxyhemoglobin oxidises NO• and NO2− to the inactive product NO3− and thus may be an important intravascular modulator of peroxidase-dependent and -independent nitration.(206)
Whilst modulation of Cl− levels is clearly not readily achievable in most situations, the levels of Br−, I− and SCN− can be modulated by dietary or other means (e.g., cessation of smoking for SCN−). Whilst lower levels of these ions can modulate the nature of the oxidants formed, the overall yield may not to be markedly affected, as HOCl formation may then predominate.
Heme poisons
Small anions, including azide and cyanide, can bind competitively to the heme centre of MPO in place of its typical halogenation substrates, thereby inhibiting hypohalous acids formation. The use of these agents is, however, limited by their multitude of interactions with other heme centres and subsequent toxicity.
MPO binding agents
Caeruloplasmin, a copper-containing plasma protein, binds MPO avidly(31) and inhibits its peroxidase and halogenation activities.(31,207) This protein may therefore limit the activity of MPO in plasma. Anti-MPO antibodies can reverse this inhibition.(208) The nature of some of these caeruloplasmin-MPO complexes has been investigated.(209) Polyanionic glycosaminoglycans, such as the anticoagulant heparin, bind (cationic) MPO electrostatically,(28) and can liberate MPO sequestered in the artery wall.(210) This interaction with MPO may exacerbate damage to heparin, or other glycosaminoglycans to which it is bound,(84,155) but divert oxidation from other critical sites. Modulation of MPO activity in this fashion could be an important, additional function of heparin in vivo. However in situations where both protein and glycosaminoglycans are present (e.g., on proteoglycans, such as the basement membrane species perlecan), damage appears to be primarily localised to the protein, with this resulting in changes to proteoglycan function.(27)
Phenols and other poor peroxidase substrates
Substrates that react readily with Compound I, thereby competitively inhibiting halide/pseudohalide ion oxidation, are a potentially attractive mechanism of inhibiting hypohalous acid formation. If such materials are poorly oxidised by Compound II, then these materials may “trap” the enzyme and prevent completion of the catalytic cycle (though see the caveats discussed above, regarding in vivo significance). The potential of this approach is however, limited by the high drug concentrations required to compete with halide oxidation. Some plasma components may exhibit this behaviour, with both ascorbate and urate oxidised by MPO;(211,212) the quantitative significance of these reactions in vivo remains to be fully established.
A number of non-steroidal anti-inflammatory drugs have been shown to inhibit the formation of HOCl (and other hypohalous acids) by MPO by acting as poor peroxidase substrates for the enzyme.(211,213) The most effective agents are likely to be those with redox potentials between 1.35 and 1.1 V, which results in significant rates of oxidation by Compound I, but not Compound II.(211,214) As O2•− can reduce Compound II to the ferric state, these compounds would be expected to be most effective (i.e. have lower IC50 values, the concentration of drug required to produce 50% inhibition) in the presence of SOD in vitro.(215) This is unlikely to happen within the neutrophil phagosome however, where the SOD concentration is limited.(11)
The common anti-inflammatory drug paracetamol (acetaminophen) reaches some of the highest plasma concentrations of any exogenous compound. Thus standard pharmacological doses (1 g) give peak plasma levels of up to 130 µM.(135) Such levels result in marked inhibition of HOCl and HOBr generation by MPO-H2O2-halide systems (IC50 77 µM for HOCl formation, 19 µM for HOBr formation, and 92 µM for both (52)). Similar inhibition of hypohalous acid formation was detected with activated neutrophils (IC50 ~ 100 µM), without perturbation of O2•− formation.(52) This inhibition is accompanied by paracetamol dimer formation as a result of oxidation of the drug to the corresponding phenoxyl radical and subsequent dimerisation;(52) these dimers may act as a useful marker of MPO inhibition by this drug.(52) Further trials of paracetamol in humans are under way.
Other phenols may behave in a similar manner, but whether these compounds achieve sufficiently high plasma concentrations to be effective in vivo is unclear. Recent studies have reported that flavanoids may act as MPO substrates, with this resulting in irreversible inactivation, and oxidation of the flavonoid to a radical and subsequent dimerization. In the presence of GSH, hydroquinone formation was detected and subsequent GSH conjugation.(216) Despite this interaction, the yield of chlorinating oxidants was not markedly affected, and therefore it was concluded that these materials were unlikely to be pharmacologically useful.(216) In contrast, quercitin, and some analogues and metabolites, has been shown to be an effective inhibitor (IC50 ~ 1 µM for quercitin) of neutrophil-mediated LDL modification, with this ascribed to inhibition of MPO-mediated oxidant formation by quercitin.(217) The effect of the flavonoids and related materials may therefore be complex and structure dependent. As many of these materials have low biological availability (i.e. peak plasma concentrations being nM or low µM(218)) very low µM IC50 values for MPO inhibition would be required for any biological effect.
As might be expected on the basis of the above data, anilines and indoles (including the amino acid tryptophan(219–221) and analogues(222)) are readily oxidized by MPO Compound I, and act as poor peroxidase substrates (i.e. result in the accumulation of Compound II, and in cases Compound III(219)). As with phenols, these compounds show lower IC50 values in the presence of SOD, as a result of the removal of O2•−.(219) The efficiencies and mechanisms of inhibition by different tryptophan analogues has been examined.(222) These studies indicate that structural changes, particularly those that increase hydrophobicity, increase the effectiveness of these materials.
An alternative approach has also been examined: rather than providing a substrate that can be metabolized to a radical by MPO (which might be damaging), stable radicals that might be metabolized to non-radical products were examined.(215) Thus long-lived nitroxide radicals, that do not give rise to overt toxicology in extended feeding studies,(223) have been examined as MPO inhibitors. A number of these compounds are potent inhibitors of MPO-mediated oxidant formation, from both isolated MPO and activated neutrophils, with IC50 values in the low µM range.(215) Evidence has been presented for Compound II accumulation with some nitroxides, but others appear to induce heme destruction.(215) The IC50 values show a marked structure dependence with positively-charged species being much more effective than negatively-charged; this has been ascribed to an interaction between the nitroxide and negatively-charged groups on the enzyme. In vivo studies are required to elucidate the true therapeutic potential of these compounds.
A number of other drugs, including hydroquinone(124) and amsacrine,(126) can also divert MPO from HOCl production via an alternative mechanism by promoting Compound III formation; again O2•− would be expected to antagonise this inhibition, by recycling this species to the native enzyme.
Hydrazines and hydrazides
The most “effective” (i.e. lowest IC50 values) inhibitors of MPO are irreversible enzyme inhibitors (’suicide’ substrates); these include hydrazines (RNHNH2) and hydrazides (RCONHNH2).(224–226) Despite their low IC50 values these compounds may not however be the most applicable in vivo. Benzoic acid hydrazides, including the most potent MPO inhibitor identified to date, 4-aminobenzoic acid hydrazide (ABAH),(128) are believed to act via heme destruction arising from generation of ferrous MPO,(224) however the precise mechanism is uncertain. Irreversible inhibition of MPO by the hydrazide derivative isoniazid, an anti-tuberculosis drug, has also been proposed to involve heme modification via a mechanism initiated by Compound III formation.(227)
Conclusions
There is now considerable evidence that MPO and other heme peroxidases are enzymes that have both beneficial and damaging actions. These species have a clear beneficial function in terms of killing invading pathogens, but this same enzymatic activity can result in host tissue damage and play a role in a number of important human pathologies. Information and an understanding of the enzymology and mechanisms of action of these enzymes is clearly therefore of major importance, both in terms of enhancing their positive actions in terms of disinfection and improved hygiene, and diminishing their deleterious effects. Recent studies have provided valuable information on the targets and actions of the oxidants generated by these enzymes and their mechanisms of biological damage. Ongoing studies on the control of the halogenation and peroxidase cycles are providing valuable data on new therapeutic strategies to limit oxidant damage to human tissues and it is possible that selective inhibition of these enzymes will have major clinical benefits.
Acknowledgments
The author is grateful for financial support of his research by Australian Research Council under the ARC Centres of Excellence (CE0561607) and Discovery (DP0988311) programs, the National Health and Medical Research Council (570829) and the National Heart Foundation (Grants in Aid: G09S4313 and G08S3769).
Abbreviations
- NOX
NADPH oxidase
- O2•−
superoxide radicals
- H2O2
hydrogen peroxide
- HO•
hydroxyl radicals
- MPO
myeloperoxidase
- NO•
nitric oxide
- ONOOH
peroxynitrite
- EPO
eosinophil peroxidase
- LPO
lactoperoxidase
- GSH
glutathione
- GSSG
glutathione disulfide
- MMP
matrix metalloproteinases
- 3-chloroTyr
3-chlorotyrosine
- MAPK
mitogen-activated protein kinase
- AGEs
advanced glycation end products
- CVD
cardiovascular disease
- 1O2
singlet oxygen
- NO+
nitrosonium ion
- DPI
diphenylene iodonium chloride
- ABAH
4-aminobenzoic acid hydrazide
References
- 1.Babior BM. The respiratory burst oxidase. TIBS. 1987;12:241–243. [Google Scholar]
- 2.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 3.Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 4.Schultz J, Kaminker K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- 5.Daugherty A, Dunn JL, Rateri DL, Heinecke JW. Myeloperoxidase, a catalyst for lipoprotein oxidation, is expressed in human atherosclerotic lesions. J Clin Invest. 1994;94:437–444. doi: 10.1172/JCI117342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malle E, Waeg G, Schreiber R, Gröne EF, Sattler WS, Gröne HJ. Immunohistochemical evidence for the myeloperoxidase/H2O2/halide system in human atherosclerotic lesions—Colocalization of myeloperoxidase and hypochlorite-modified proteins. Eur J Biochem. 2000;267:4495–4503. doi: 10.1046/j.1432-1327.2000.01498.x. [DOI] [PubMed] [Google Scholar]
- 7.Nauseef WM. Myeloperoxidase deficiency. Hematol Oncol Clin North Am. 1988;2:135–158. [PubMed] [Google Scholar]
- 8.Kutter D, Devaquet P, Vanderstocken G, Paulus JM, Marchal V, Gothot A. Consequences of total and subtotal myeloperoxidase deficiency: risk or benefit? Acta Haematol. 2000;104:10–15. doi: 10.1159/000041062. [DOI] [PubMed] [Google Scholar]
- 9.Klebanoff SJ. Myeloperoxidase-halide-hydrogen peroxide antibacterial system. J Bacteriol. 1968;95:2131–2138. doi: 10.1128/jb.95.6.2131-2138.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies MJ, Hawkins CL, Pattison DI, Rees MD. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 11.Kettle AJ, Winterbourn CC. Myeloperoxidase: a key regulator of neutrophil oxidant production. Redox Rep. 1997;3:3–15. doi: 10.1080/13510002.1997.11747085. [DOI] [PubMed] [Google Scholar]
- 12.van der Veen BS, de Winther MP, Heeringa P. Myeloperoxidase: molecular mechanisms of action and their relevance to human health and disease. Antioxid Redox Signal. 2009;11:2899–2937. doi: 10.1089/ars.2009.2538. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler TJ, Davey CA, Fenna RE. X-ray crystal structure and characterization of halide-binding sites of human myeloperoxidase at 1.8 A resolution. J Biol Chem. 2000;275:11964–11971. doi: 10.1074/jbc.275.16.11964. [DOI] [PubMed] [Google Scholar]
- 14.Zeng J, Fenna RE. X-ray crystal structure of canine myeloperoxidase at 3 A resolution. J Mol Biol. 1992;226:185–207. doi: 10.1016/0022-2836(92)90133-5. [DOI] [PubMed] [Google Scholar]
- 15.Bolscher BG, Wever R. A kinetic study of the reaction between human myeloperoxidase, hydroperoxides and cyanide. Inhibition by chloride and thiocyanate. Biochim Biophys Acta. 1984;788:1–10. doi: 10.1016/0167-4838(84)90290-5. [DOI] [PubMed] [Google Scholar]
- 16.Furtmüller PG, Zederbauer M, Jantschko W, et al. Active site structure and catalytic mechanisms of human peroxidases. Arch Biochem Biophys. 2006;445:199–213. doi: 10.1016/j.abb.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 17.Abu-Ghazaleh RI, Dunnette SL, Loegering DA, et al. Eosinophil granule proteins in peripheral blood granulocytes. J Leukoc Biol. 1992;52:611–618. doi: 10.1002/jlb.52.6.611. [DOI] [PubMed] [Google Scholar]
- 18.Ten RM, Pease LR, McKean DJ, Bell MP, Gleich GJ. Molecular cloning of the human eosinophil peroxidase. Evidence for the existence of a peroxidase multigene family. J Exp Med. 1989;169:1757–1769. doi: 10.1084/jem.169.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ihalin R, Loimaranta V, Tenovuo J. Origin, structure, and biological activities of peroxidases in human saliva. Arch Biochem Biophys. 2006;445:261–268. doi: 10.1016/j.abb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 20.Ueda T, Sakamaki K, Kuroki T, Yano I, Nagata S. Molecular cloning and characterization of the chromosomal gene for human lactoperoxidase. Eur J Biochem. 1997;243:32–41. doi: 10.1111/j.1432-1033.1997.0032a.x. [DOI] [PubMed] [Google Scholar]
- 21.Miyasaki KT, Zambon JJ, Jones CA, Wilson ME. Role of high avidity binding of human neutrophil myeloperoxidase in the killing of Actinobacillus actinomycetemcomitans. Infect Immun. 1987;55:1029–1036. doi: 10.1128/iai.55.5.1029-1036.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Selvaraj RJ, Zgliczynski JM, Paul BB, Sbarra AJ. Enhanced killing of myeloperoxidase-coated bacteria in the myeloperoxidase-H2O2-Cl-system. J Infect Dis. 1978;137:481–485. doi: 10.1093/infdis/137.4.481. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey PG, Martin T, Chi E, Klebanoff SJ. Arming of mononuclear phagocytes by eosinophil peroxidase bound to Staphylococcus aureus. J Immunol. 1982;128:415–420. [PubMed] [Google Scholar]
- 24.Baldus S, Eiserich JP, Mani A, et al. Endothelial transcytosis of myeloperoxidase confers specificity to vascular ECM proteins as targets of tyrosine nitration. J Clin Invest. 2001;108:1759–1770. doi: 10.1172/JCI12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Astern JM, Pendergraft WF, Falk RJ, et al. Myeloperoxidase interacts with endothelial cell-surface cytokeratin 1 and modulates bradykinin production by the plasma kallikrein-kinin system. Am J Pathol. 2007;171:349–360. doi: 10.2353/ajpath.2007.060831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubala L, Baldus S, Eiserich JP. Glycosaminoglycan-dependent sequestration of myeloperoxidase within extracellular matrix. Free Radic Biol Med. 2004;37(Suppl. 1):S52. [Google Scholar]
- 27.Rees MD, Whitelock JM, Malle E, et al. Myeloperoxidase-derived oxidants selectively disrupt the protein core of the heparan sulfate proteoglycan perlecan. Matrix Biol. 2010;29:63–73. doi: 10.1016/j.matbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daphna EM, Michaela S, Eynat P, Irit A, Rimon S. Association of myeloperoxidase with heparin: oxidative inactivation of proteins on the surface of endothelial cells by the bound enzyme. Mol Cell Biochem. 1998;183:55–61. doi: 10.1023/a:1006848730927. [DOI] [PubMed] [Google Scholar]
- 29.Green SP, Baker MS, Lowther DA. Depolymerization of synovial fluid hyaluronic acid (HA) by the complete myeloperoxidase (MPO) system may involve the formation of a HA-MPO ionic complex. J Rheumatol. 1990;17:1670–1675. [PubMed] [Google Scholar]
- 30.Tiruppathi C, Naqvi T, Wu Y, Vogel SM, Minshall RD, Malik AB. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc Natl Acad Sci USA. 2004;101:7699–7704. doi: 10.1073/pnas.0401712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segelmark M, Persson B, Hellmark T, Wieslander J. Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin Exp Immunol. 1997;108:167–174. doi: 10.1046/j.1365-2249.1997.d01-992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouriche H, Salavei P, Lessig J, Arnhold J. Differential effects of flavonols on inactivation of alpha1-antitrypsin induced by hypohalous acids and the myeloperoxidase-hydrogen peroxide-halide system. Arch Biochem Biophys. 2007;459:137–142. doi: 10.1016/j.abb.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Zheng L, Nukuna B, Brennan ML, et al. Apolipoprotein A-I is a selective target for myeloperoxidase-catalyzed oxidation and functional impairment in subjects with cardiovascular disease. J Clin Invest. 2004;114:529–541. doi: 10.1172/JCI21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carr AC, Myzak MC, Stocker R, McCall MR, Frei B. Myeloperoxidase binds to low-density lipoprotein: potential implications for atherosclerosis. FEBS Lett. 2000;487:176–180. doi: 10.1016/s0014-5793(00)02227-4. [DOI] [PubMed] [Google Scholar]
- 35.Zederbauer M, Furtmüller PG, Brogioni S, Jakopitsch C, Smulevich G, Obinger C. Heme to protein linkages in mammalian peroxidases: impact on spectroscopic, redox and catalytic properties. Nat Prod Rep. 2007;24:571–584. doi: 10.1039/b604178g. [DOI] [PubMed] [Google Scholar]
- 36.Spalteholz H, Panasenko OM, Arnhold J. Formation of reactive halide species by myeloperoxidase and eosinophil peroxidase. Arch Biochem Biophys. 2006;445:225–234. doi: 10.1016/j.abb.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Marquez LA, Dunford HB. Reaction of compound-III of myeloperoxidase with ascorbic acid. J Biol Chem. 1990;265:6074–6078. [PubMed] [Google Scholar]
- 38.Marquez LA, Dunford HB. Interaction of acetaminophen with myeloperoxidase intermediates: optimum stimulation of enzyme activity. Arch Biochem Biophys. 1993;305:414–420. doi: 10.1006/abbi.1993.1440. [DOI] [PubMed] [Google Scholar]
- 39.Kettle AJ, Anderson RF, Hampton MB, Winterbourn CC. Reactions of superoxide with myeloperoxidase. Biochemistry. 2007;46:4888–4897. doi: 10.1021/bi602587k. [DOI] [PubMed] [Google Scholar]
- 40.Arnhold J, Monzani E, Furtmüller PG, Zederbauer M, Casella L, Obinger C. Kinetics and thermodynamics of halide and nitrite oxidation by mammalian heme peroxidases. Eur J Inorg Chem. 2006;19:3801–3811. [Google Scholar]
- 41.van Dalen CJ, Whitehouse MW, Winterbourn CC, Kettle AJ. Thiocyanate and chloride as competing substrates for myeloperoxidase. Biochem J. 1997;327:487–492. doi: 10.1042/bj3270487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chapman AL, Skaff O, Senthilmohan R, Kettle AJ, Davies MJ. Hypobromous acid and bromamine production by neutrophils and modulation by superoxide. Biochem J. 2009;417:773–781. doi: 10.1042/BJ20071563. [DOI] [PubMed] [Google Scholar]
- 43.van Dalen CJ, Kettle AJ. Substrates and products of eosinophil peroxidase. Biochem J. 2001;358:233–239. doi: 10.1042/0264-6021:3580233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Furtmüller PG, Jantschko W, Regelsberger G, Jakopitsch C, Arnhold J, Obinger C. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry. 2002;41:11895–11900. doi: 10.1021/bi026326x. [DOI] [PubMed] [Google Scholar]
- 45.Ashby MT, Carlson AC, Scott MJ. Redox buffering of hypochlorous acid by thiocyanate in physiologic fluids. J Am Chem Soc. 2004;126:15976–15977. doi: 10.1021/ja0438361. [DOI] [PubMed] [Google Scholar]
- 46.Nagy P, Beal JL, Ashby MT. Thiocyanate is an efficient endogenous scavenger of the phagocytic killing agent hypobromous acid. Chem Res Toxicol. 2006;19:587–593. doi: 10.1021/tx050338c. [DOI] [PubMed] [Google Scholar]
- 47.Morris JC. The acid ionization constant of HOCl from 5°C to 35°C. J Phys Chem. 1966;70:3798–3805. [Google Scholar]
- 48.Prütz WA, Kissner R, Koppenol WH, Rüegger H. On the irreversible destruction of reduced nicotinamide nucleotides by hypohalous acids. Arch Biochem Biophys. 2000;380:181–191. doi: 10.1006/abbi.2000.1914. [DOI] [PubMed] [Google Scholar]
- 49.Nagy P, Jameson GN, Winterbourn CC. Kinetics and mechanisms of the reaction of hypothiocyanous acid with 5-thio-2-nitrobenzoic acid and reduced glutathione. Chem Res Toxicol. 2009;22:1833–1840. doi: 10.1021/tx900249d. [DOI] [PubMed] [Google Scholar]
- 50.Ikeda-Saito M. A study of ligand binding to spleen myeloperoxidase. Biochemistry. 1987;26:4344–4349. doi: 10.1021/bi00388a024. [DOI] [PubMed] [Google Scholar]
- 51.Marquez LA, Dunford HB. Chlorination of taurine by myeloperoxidase. Kinetic evidence for an enzyme-bound intermediate. J Biol Chem. 1994;269:7950–7956. [PubMed] [Google Scholar]
- 52.Koelsch M, Mallak R, Graham GG, et al. Acetaminophen (paracetamol) inhibits myeloperoxidase-catalyzed oxidant production and biological damage at therapeutically achievable concentrations. Biochem Pharmacol. 2010;79:1156–1164. doi: 10.1016/j.bcp.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 53.Furtmüller PG, Arnhold J, Jantschko W, Pichler H, Obinger C. Redox properties of the couples compound I/compound II and compound II/native enzyme of human myeloperoxidase. Biochem Biophys Res Commun. 2003;301:551–557. doi: 10.1016/s0006-291x(02)03075-9. [DOI] [PubMed] [Google Scholar]
- 54.Furtmüller PG, Arnhold J, Jantschko W, Zederbauer M, Jakopitsch C, Obinger C. Standard reduction potentials of all couples of the peroxidase cycle of lactoperoxidase. J Inorg Biochem. 2005;99:1220–1229. doi: 10.1016/j.jinorgbio.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 55.Marquez LA, Dunford HB. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II. Implications for lipoprotein peroxidation studies. J Biol Chem. 1995;270:30434–30440. doi: 10.1074/jbc.270.51.30434. [DOI] [PubMed] [Google Scholar]
- 56.Bayse GS, Michaels AW, Morrison M. The peroxidase catalyzed oxidation of tyrosine. Biochim Biophys Acta. 1972;284:34–42. doi: 10.1016/0005-2744(72)90043-5. [DOI] [PubMed] [Google Scholar]
- 57.Pattison DI, Davies MJ. Absolute rate constants for the reaction of hypochlorous acid with protein side-chains and peptide bonds. Chem Res Toxicol. 2001;14:1453–1464. doi: 10.1021/tx0155451. [DOI] [PubMed] [Google Scholar]
- 58.Pattison DI, Davies MJ. Reactions of myeloperoxidase-derived oxidants with biological substrates: Gaining chemical insight into human inflammatory diseases. Curr Med Chem. 2006;13:3271–3290. doi: 10.2174/092986706778773095. [DOI] [PubMed] [Google Scholar]
- 59.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: absolute rate constants, product analysis and computational modeling. Chem Res Toxicol. 2003;16:439–449. doi: 10.1021/tx025670s. [DOI] [PubMed] [Google Scholar]
- 60.Carr AC, Winterbourn CC. Oxidation of neutrophil glutathione and protein thiols by myeloperoxidase-derived hypochlorous acid. Biochem J. 1997;327:275–281. doi: 10.1042/bj3270275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davies MJ, Hawkins CL. Hypochlorite-induced oxidation of thiols: formation of thiyl radicals and the role of sulfenyl chlorides as intermediates. Free Radic Res. 2000;33:719–729. doi: 10.1080/10715760000301241. [DOI] [PubMed] [Google Scholar]
- 62.Vissers MC, Winterbourn CC. Oxidation of intracellular glutathione after exposure of human red blood cells to hypochlorous acid. Biochem J. 1995;307:57–62. doi: 10.1042/bj3070057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lloyd MM, van Reyk DM, Davies MJ, Hawkins CL. Hypothiocyanous acid is a more potent inducer of apoptosis and protein thiol depletion in murine macrophage cells than hypochlorous acid or hypobromous acid. Biochem J. 2008;414:271–280. doi: 10.1042/BJ20080468. [DOI] [PubMed] [Google Scholar]
- 64.Hawkins CL, Pattison DI, Davies MJ. Hypochlorite-induced oxidation of amino acids, peptides and proteins. Amino Acids. 2003;25:259–274. doi: 10.1007/s00726-003-0016-x. [DOI] [PubMed] [Google Scholar]
- 65.Fu X, Mueller DM, Heinecke JW. Generation of intramolecular and intermolecular sulfenamides, sulfinamides, and sulfonamides by hypochlorous acid: a potential pathway for oxidative cross-linking of low-density lipoprotein by myeloperoxidase. Biochemistry. 2002;41:1293–1301. doi: 10.1021/bi015777z. [DOI] [PubMed] [Google Scholar]
- 66.Harwood DT, Kettle AJ, Winterbourn CC. Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC-MS/MS method. Biochem J. 2006;399:161–168. doi: 10.1042/BJ20060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raftery MJ, Yang Z, Valenzuela SM, Geczy CL. Novel intra- and inter-molecular sulfinamide bonds in S100A8 produced by hypochlorite oxidation. J Biol Chem. 2001;276:33393–33401. doi: 10.1074/jbc.M101566200. [DOI] [PubMed] [Google Scholar]
- 68.Pullar JM, Vissers MC, Winterbourn CC. Glutathione oxidation by hypochlorous acid in endothelial cells produces glutathione sulfonamide as a major product but not glutathione disulfide. J Biol Chem. 2001;276:22120–22125. doi: 10.1074/jbc.M102088200. [DOI] [PubMed] [Google Scholar]
- 69.Harwood DT, Kettle AJ, Brennan S, Winterbourn CC. Simultaneous determination of reduced glutathione, glutathione disulphide and glutathione sulphonamide in cells and physiological fluids by isotope dilution liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:3393–3399. doi: 10.1016/j.jchromb.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 70.Pullar JM, Winterbourn CC, Vissers MC. Loss of GSH and thiol enzymes in endothelial cells exposed to sublethal concentrations of hypochlorous acid. Am J Physiol. 1999;277:H1505–H1512. doi: 10.1152/ajpheart.1999.277.4.H1505. [DOI] [PubMed] [Google Scholar]
- 71.Fu X, Kassim SY, Parks WC, Heinecke JW. Hypochlorous acid oxygenates the cysteine switch domain of pro-matrilysin (MMP-7). A mechanism for matrix metalloproteinase activation and atherosclerotic plaque rupture by myeloperoxidase. J Biol Chem. 2001;276:41279–41287. doi: 10.1074/jbc.M106958200. [DOI] [PubMed] [Google Scholar]
- 72.Hawkins CL, Davies MJ. Inactivation of protease inhibitors and lysozyme by hypochlorous acid: role of side-chain oxidation and protein unfolding in loss of biological function. Chem Res Toxicol. 2005;18:1600–1610. doi: 10.1021/tx050207b. [DOI] [PubMed] [Google Scholar]
- 73.Pattison DI, Hawkins CL, Davies MJ. Hypochlorous acid-mediated protein oxidation: How important are chloramine transfer reactions and protein tertiary structure? Biochemistry. 2007;46:9853–9864. doi: 10.1021/bi7008294. [DOI] [PubMed] [Google Scholar]
- 74.Kim HY, Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J. 2007;407:321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- 75.Matheson NR, Travis J. Differential effects of oxidizing agents on human plasma alpha-1-proteinase inhibitor and human neutrophil myeloperoxidase. Biochemistry. 1985;24:1941–1945. doi: 10.1021/bi00329a021. [DOI] [PubMed] [Google Scholar]
- 76.Vissers MC, Winterbourn CC. Myeloperoxidase-dependent oxidative inactivation of neutrophil neutral proteinases and microbicidal enzymes. Biochem J. 1987;245:277–280. doi: 10.1042/bj2450277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khor HK, Fisher MT, Schöneich C. Potential role of methionine sulfoxide in the inactivation of the chaperone GroEL by hypochlorous acid (HOCl) and peroxynitrite (ONOO-) J Biol Chem. 2004;279:19486–19493. doi: 10.1074/jbc.M310045200. [DOI] [PubMed] [Google Scholar]
- 78.Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, Winterbourn CC. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem J. 2006;396:71–78. doi: 10.1042/BJ20052026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas EL. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chlorine derivatives of bacterial components in bactericidal action against Escherichia coli. Infect Immun. 1979;23:522–531. doi: 10.1128/iai.23.2.522-531.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winterbourn CC. Comparative reactivities of various biological compounds with myeloperoxidase-hydrogen peroxide-chloride, and similarity of the oxidant to hypochlorite. Biochim Biophys Acta. 1985;840:204–210. doi: 10.1016/0304-4165(85)90120-5. [DOI] [PubMed] [Google Scholar]
- 81.Thomas EL, Grisham MB, Jefferson MM. Preparation and characterization of chloramines. Method Enzymol. 1986;132:569–585. doi: 10.1016/s0076-6879(86)32042-1. [DOI] [PubMed] [Google Scholar]
- 82.Prütz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys. 1996;332:110–120. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 83.Prütz WA. Interactions of hypochlorous acid with pyrimidine nucleotides, and secondary reactions of chlorinated pyrimidines with GSH, NADH, and other substrates. Arch Biochem Biophys. 1998;349:183–191. doi: 10.1006/abbi.1997.0440. [DOI] [PubMed] [Google Scholar]
- 84.Rees MD, Hawkins CL, Davies MJ. Hypochlorite-mediated fragmentation of hyaluronan, chondritin sulfates, and related N-acetyl glycosamines: evidence for chloramide intermediates, free radical transfer reactios, and site-specific fragmentation. J Am Chem Soc. 2003;125:13719–13733. doi: 10.1021/ja0370591. [DOI] [PubMed] [Google Scholar]
- 85.Kawai Y, Kiyokawa H, Kimura Y, Kato Y, Tsuchiya K, Terao J. Hypochlorous acid-derived modification of phospholipids: characterization of aminophospholipids as regulatory molecules for lipid peroxidation. Biochemistry. 2006;45:14201–14211. doi: 10.1021/bi0610909. [DOI] [PubMed] [Google Scholar]
- 86.Kettle AJ. Neutrophils convert tyrosyl residues in albumin to chlorotyrosine. FEBS Lett. 1996;379:103–106. doi: 10.1016/0014-5793(95)01494-2. [DOI] [PubMed] [Google Scholar]
- 87.Winterbourn CC. Biological reactivity and biomarkers of the neutrophil oxidant, hypochlorous acid. Toxicology. 2002;181–182:223–227. doi: 10.1016/s0300-483x(02)00286-x. [DOI] [PubMed] [Google Scholar]
- 88.Fu X, Kao JL, Bergt C, et al. Oxidative cross-linking of tryptophan to glycine restrains matrix metalloproteinase activity: Specific structural motifs control protein oxidation. J Biol Chem. 2004;279:6209–6212. doi: 10.1074/jbc.C300506200. [DOI] [PubMed] [Google Scholar]
- 89.Gould JP, Richards JT, Miles MG. The formation of stable organic chloramines during the aqueous chlorination of cytosine and 5-methylcytosine. Water Res. 1984;18:991–999. [Google Scholar]
- 90.Henderson JP, Byun J, Williams MV, et al. Bromination of deoxycytidine by eosinophil peroxidase: a mechanism for mutagenesis by oxidative damage of nucleotide precursors. Proc Natl Acad Sci USA. 2001;98:1631–1636. doi: 10.1073/pnas.041146998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henderson JP, Byun J, Williams MV, Mueller DM, McCormick ML, Heinecke JW. Production of brominating intermediates by myeloperoxidase. A transhalogenation pathway for generating mutagenic nucleobases during inflammation. J Biol Chem. 2001;276:7867–7875. doi: 10.1074/jbc.M005379200. [DOI] [PubMed] [Google Scholar]
- 92.Henderson JP, Byun J, Takeshita J, Heinecke JW. Phagocytes produce 5-chlorouracil and 5-bromouracil, two mutagenic products of myeloperoxidase, in human inflammatory tissue. J Biol Chem. 2003;278:23522–23528. doi: 10.1074/jbc.M303928200. [DOI] [PubMed] [Google Scholar]
- 93.Henderson JP, Byun J, Mueller DM, Heinecke JW. The eosinophil peroxidase-hydrogen peroxide-bromide system of human eosinophils generates 5-bromouracil, a mutagenic thymine analogue. Biochemistry. 2001;40:2052–2059. doi: 10.1021/bi002015f. [DOI] [PubMed] [Google Scholar]
- 94.Masuda M, Suzuki T, Friesen MD, et al. Chlorination of guanosine and other nucleosides by hypochlorous acid and myeloperoxidase of activated human neutrophils. Catalysis by nicotine and trimethylamine. J Biol Chem. 2001;276:40486–40496. doi: 10.1074/jbc.M102700200. [DOI] [PubMed] [Google Scholar]
- 95.Chen HJ, Row SW, Hong CL. Detection and quantification of 5-chlorocytosine in DNA by stable isotope dilution and gas chromatography/negative ion chemical ionization/mass spectrometry. Chem Res Toxicol. 2002;15:262–268. doi: 10.1021/tx015578g. [DOI] [PubMed] [Google Scholar]
- 96.Whiteman M, Jenner A, Halliwell B. Hypochlorous acid-induced base modifications in isolated calf thymus DNA. Chem Res Toxicol. 1997;10:1240–1246. doi: 10.1021/tx970086i. [DOI] [PubMed] [Google Scholar]
- 97.Whiteman M, Jenner A, Halliwell B. 8-Chloroadenine: a novel product formed from hypochlorous acid-induced damage to calf thymus DNA. Biomarkers. 1999;4:303–310. doi: 10.1080/135475099230831. [DOI] [PubMed] [Google Scholar]
- 98.Shen Z, Mitra SN, Wu W, et al. Eosinophil peroxidase catalyzes bromination of free nucleosides and double-stranded DNA. Biochemistry. 2001;40:2041–2051. doi: 10.1021/bi001961t. [DOI] [PubMed] [Google Scholar]
- 99.Kawai Y, Morinaga H, Kondo H, et al. Endogenous formation of novel halogenated 2'-deoxycytidine. Hypohalous acid-mediated DNA modification at the site of inflammation. J Biol Chem. 2004;279:51241–51249. doi: 10.1074/jbc.M408210200. [DOI] [PubMed] [Google Scholar]
- 100.Takeshita J, Byun J, Nhan TQ, et al. Myeloperoxidase generates 5-chlorouracil in human atherosclerotic tissue. A potential pathway for somatic mutagenesis by macrophages. J Biol Chem. 2006;281:3096–3104. doi: 10.1074/jbc.M509236200. [DOI] [PubMed] [Google Scholar]
- 101.van den Berg JJ, Winterbourn CC, Kuypers FA. Hypochlorous acid-mediated modification of cholesterol and phospholipid: analysis of reaction products by gas chromatography-mass spectrometry. J Lipid Res. 1993;34:2005–2012. [PubMed] [Google Scholar]
- 102.Arnhold J, Panasenko OM, Schiller J, Vladimirov Yu A, Arnold K. The action of hypochlorous acid on phosphatidylcholine liposomes in dependence on the content of double bonds. Stoichiometry and NMR analysis. Chem Phys Lipids. 1995;78:55–64. doi: 10.1016/0009-3084(95)02484-z. [DOI] [PubMed] [Google Scholar]
- 103.Panasenko OM, Spalteholz H, Schiller J, Arnhold J. Myeloperoxidase-induced formation of chlorohydrins and lysophospholipids from unsaturated phosphatidylcholines. Free Radic Biol Med. 2003;34:553–562. doi: 10.1016/s0891-5849(02)01358-8. [DOI] [PubMed] [Google Scholar]
- 104.Carr AC, van den Berg JJ, Winterbourn CC. Chlorination of cholesterol in cell membranes by hypochlorous acid. Arch Biochem Biophys. 1996;332:63–69. doi: 10.1006/abbi.1996.0317. [DOI] [PubMed] [Google Scholar]
- 105.Carr AC, van den Berg JJ, Winterbourn CC. Differential reactivities of hypochlorous and hypobromous acids with purified Escherichia coli phospholipid: formation of haloamines and halohydrins. Biochim Biophys Acta. 1998;1392:254–264. doi: 10.1016/s0005-2760(98)00038-1. [DOI] [PubMed] [Google Scholar]
- 106.Jerlich A, Pitt AR, Schaur RJ, Spickett CM. Pathways of phospholipid oxidation by HOCl in human LDL detected by LC-MS. Free Radic Biol Med. 2000;28:673–682. doi: 10.1016/s0891-5849(99)00273-7. [DOI] [PubMed] [Google Scholar]
- 107.Heinecke JW, Li W, Mueller DM, Bohrer A, Turk J. Cholesterol chlorohydrin synthesis by the myeloperoxidase-hydrogen peroxide-chloride system: potential markers for lipoproteins oxidatively damaged by phagocytes. Biochemistry. 1994;33:10127–10136. doi: 10.1021/bi00199a041. [DOI] [PubMed] [Google Scholar]
- 108.Skaff O, Pattison DI, Davies MJ. The vinyl ether linkages of plasmalogens are favored targets for myeloperoxidase-derived oxidants: a kinetic study. Biochemistry. 2008;47:8237–8245. doi: 10.1021/bi800786q. [DOI] [PubMed] [Google Scholar]
- 109.Albert CJ, Thukkani AK, Heuertz RM, Slungaard A, Hazen SL, Ford DA. Eosinophil peroxidase-derived reactive brominating species target the vinyl ether bond of plasmalogens generating a novel chemoattractant, alpha-bromo fatty aldehyde. J Biol Chem. 2003;278:8942–8950. doi: 10.1074/jbc.m211634200. [DOI] [PubMed] [Google Scholar]
- 110.Thukkani AK, Albert CJ, Wildsmith KR, et al. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J Biol Chem. 2003;278:36365–36372. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- 111.Thukkani AK, McHowat J, Hsu FF, Brennan ML, Hazen SL, Ford DA. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 112.Pattison DI, Davies MJ. Kinetic analysis of the reactions of hypobromous acid with protein components: implications for cellular damage and the use of 3-bromotyrosine as a marker of oxidative stress. Biochemistry. 2004;43:4799–4809. doi: 10.1021/bi035946a. [DOI] [PubMed] [Google Scholar]
- 113.Skaff O, Pattison DI, Davies MJ. Kinetics of hypobromous acid-mediated oxidation of lipid components and antioxidants. Chem Res Toxicol. 2007;20:1980–1988. doi: 10.1021/tx7003097. [DOI] [PubMed] [Google Scholar]
- 114.Vissers MC, Carr AC, Chapman AL. Comparison of human red cell lysis by hypochlorous and hypobromous acids: insights into the mechanism of lysis. Biochem J. 1998;330:131–138. doi: 10.1042/bj3300131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hawkins CL. The role of hypothiocyanous acid (HOSCN) in biological systems. Free Rad Res. 2009;43:1147–1158. doi: 10.3109/10715760903214462. [DOI] [PubMed] [Google Scholar]
- 116.Saloojee Y, Vesey CJ, Cole PV, Russell MA. Carboxyhaemoglobin and plasma thiocyanate: complementary indicators of smoking behaviour? Thorax. 1982;37:521–525. doi: 10.1136/thx.37.7.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang Z, Nicholls SJ, Rodriguez ER, et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 118.Pattison DI, Hawkins CL, Davies MJ. What are the plasma targets of the oxidant hypochlorous acid? A kinetic modeling approach. Chem Res Toxicol. 2009;22:807–817. doi: 10.1021/tx800372d. [DOI] [PubMed] [Google Scholar]
- 119.Skaff O, Pattison DI, Davies MJ. Hypothiocyanous acid reactivity with low-molecular-mass and protein thiols: Absolute rate constants and assessment of biological relevance. Biochem J. 2009;422:111–117. doi: 10.1042/BJ20090276. [DOI] [PubMed] [Google Scholar]
- 120.Hawkins CL, Pattison DI, Stanley NR, Davies MJ. Tryptophan residues are targets in hypothiocyanous acid-mediated protein oxidation. Biochem J. 2008;416:441–452. doi: 10.1042/BJ20070941. [DOI] [PubMed] [Google Scholar]
- 121.Aune TM, Thomas EL, Morrison M. Lactoperoxidase-catalyzed incorporation of thiocyanate ion into a protein substrate. Biochemistry. 1977;16:4611–4615. doi: 10.1021/bi00640a013. [DOI] [PubMed] [Google Scholar]
- 122.Lane AE, Tan JT, Hawkins CL, Heather AK, Davies MJ. The myeloperoxidase-derived oxidant HOSCN inhibits protein tyrosine phosphatases and modulates cell signalling via the mitogen-activated protein kinase (MAPK) pathway in macrophages. Biochem J. 2010;430:161–169. doi: 10.1042/BJ20100082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Exner M, Hermann M, Hofbauer R, Hartmann B, Kapiotis S, Gmeiner B. Thiocyanate catalyzes myeloperoxidase-initiated lipid oxidation in LDL. Free Radic Biol Med. 2004;37:146–155. doi: 10.1016/j.freeradbiomed.2004.04.039. [DOI] [PubMed] [Google Scholar]
- 124.Kettle AJ, Winterbourn CC. Oxidation of hydroquinone by myeloperoxidase. Mechanism of stimulation by benzoquinone. J Biol Chem. 1992;267:8319–8324. [PubMed] [Google Scholar]
- 125.Burner U, Krapfenbauer G, Furtmüller PG, Regelsberger G, Obinger C. Oxidation of hydroquinone, 2,3-dimethylhydroquinone and 2,3,5-trimethylhydroquinone by human myeloperoxidase. Redox Rep. 2000;5:185–190. doi: 10.1179/135100000101535735. [DOI] [PubMed] [Google Scholar]
- 126.Kettle AJ, Robertson IG, Palmer BD, Anderson RF, Patel KB, Winterbourn CC. Oxidative metabolism of amsacrine by the neutrophil enzyme Myeloperoxidase. Biochem Pharmacol. 1992;44:1731–1738. doi: 10.1016/0006-2952(92)90066-r. [DOI] [PubMed] [Google Scholar]
- 127.van der Walt BJ, van Zyl JM, Kriegler A. Aromatic hydroxylation during the myeloperoxidase-oxidase oxidation of hydrazines. Biochem Pharmacol. 1994;47:1039–1046. doi: 10.1016/0006-2952(94)90415-4. [DOI] [PubMed] [Google Scholar]
- 128.Kettle AJ, Geyde CA, Winterbourn CC. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic hydrazide. Biochem J. 1997;321:503–508. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siraki AG, Bonini MG, Jiang J, Ehrenshaft M, Mason RP. Aminoglutethimide-induced protein free radical formation on myeloperoxidase: a potential mechanism of agranulocytosis. Chem Res Toxicol. 2007;20:1038–1045. doi: 10.1021/tx6003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Colas C, Ortiz de Montellano PR. Autocatalytic radical reactions in physiological prosthetic heme modification. Chem Rev. 2003;103:2305–2332. doi: 10.1021/cr0204303. [DOI] [PubMed] [Google Scholar]
- 131.Colas C, De Montellano PR. Horseradish peroxidase mutants that autocatalytically modify their prosthetic heme group: insights into mammalian peroxidase heme-protein covalent bonds. J Biol Chem. 2004;279:24131–24140. doi: 10.1074/jbc.M401687200. [DOI] [PubMed] [Google Scholar]
- 132.Savenkova ML, Mueller DM, Heinecke JW. Tyrosyl radical generated by myeloperoxidase is a physiological catalyst for the initiation of lipid-peroxidation in low density lipoprotein. J Biol Chem. 1994;269:20394–20400. [PubMed] [Google Scholar]
- 133.Kapiotis S, Sengoelge G, Hermann M, Held I, Seelos C, Gmeiner BM. Paracetamol catalyzes myeloperoxidase-initiated lipid oxidation in LDL. Arterioscler Thromb Vasc Biol. 1997;17:2855–2860. doi: 10.1161/01.atv.17.11.2855. [DOI] [PubMed] [Google Scholar]
- 134.Heinecke JW, Li W, Francis GA, Goldstein JA. Tyrosyl radical generated by myeloperoxidase catalyzes the oxidative cross-linking of proteins. J Clin Invest. 1993;91:2866–2872. doi: 10.1172/JCI116531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bessems JG, Vermeulen NP. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit Rev Toxicol. 2001;31:55–138. doi: 10.1080/20014091111677. [DOI] [PubMed] [Google Scholar]
- 136.O’Brien P. Peroxidases. Chem Biol Interact. 2000;129:113–139. doi: 10.1016/s0009-2797(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 137.Tafazoli S, O’Brien PJ. Peroxidases: a role in the metabolism and side effects of drugs. Drug Discov Today. 2005;10:617–625. doi: 10.1016/S1359-6446(05)03394-5. [DOI] [PubMed] [Google Scholar]
- 138.Grisham MB, Jefferson MM, Thomas EL. Role of monochloramine in the oxidation of erythrocyte hemoglobin by stimulated neutrophils. J Biol Chem. 1984;259:6757–6765. [PubMed] [Google Scholar]
- 139.Peskin AV, Midwinter RG, Harwood DT, Winterbourn CC. Chlorine transfer between glycine, taurine and histamine: reaction rates and impact on cellular reactivity. Free Radic Biol Med. 2004;37:1622–1630. doi: 10.1016/j.freeradbiomed.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 140.Peskin AV, Winterbourn CC. Histamine chloramine reactivity with thiol compounds, ascorbate and methionine and with intracellular glutathione. Free Radic Biol Med. 2003;35:1252–1260. doi: 10.1016/s0891-5849(03)00502-1. [DOI] [PubMed] [Google Scholar]
- 141.Peskin AV, Winterbourn CC. Taurine chloramine is more selective than hypochlorous acid at targeting critical cysteines and inactivating creatine kinase and glyceraldehyde-3-phosphate dehydrogenase. Free Radic Biol Med. 2006;40:45–53. doi: 10.1016/j.freeradbiomed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 142.Thomas EL, Grisham MB, Jefferson MM. Cytotoxicity of chloramines. Method Enzymol. 1986;132:585–593. doi: 10.1016/s0076-6879(86)32043-3. [DOI] [PubMed] [Google Scholar]
- 143.Thomas EL, Bozeman PM, Jefferson MM, King CC. Oxidation of bromide by the human leukocyte enzymes myeloperoxidase and eosinophil peroxidase. Formation of bromamines. J Biol Chem. 1995;270:2906–2913. doi: 10.1074/jbc.270.7.2906. [DOI] [PubMed] [Google Scholar]
- 144.Pattison DI, Davies MJ. Kinetic analysis of the role of histidine chloramines in hypochlorous acid mediated protein oxidation. Biochemistry. 2005;44:7378–7387. doi: 10.1021/bi0474665. [DOI] [PubMed] [Google Scholar]
- 145.Pattison DI, Davies MJ. Evidence for rapid inter- and intra-molecular chlorine transfer reactions of histamine and carnosine chloramines: implications for the prevention of hypochlorous acid mediated damage. Biochemistry. 2006;45:8152–8162. doi: 10.1021/bi060348s. [DOI] [PubMed] [Google Scholar]
- 146.Hawkins CL, Davies MJ. Hypochlorite-induced damage to proteins: formation of nitrogen-centred radicals from lysine residues and their role in protein fragmentation. Biochem J. 1998;332:617–625. doi: 10.1042/bj3320617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hawkins CL, Davies MJ. Hypochlorite-induced oxidation of proteins in plasma: formation of chloramines and nitrogen-centred radicals and their role in protein fragmentation. Biochem J. 1999;340:539–548. [PMC free article] [PubMed] [Google Scholar]
- 148.Armesto XL, Canle ML, Garcia MV, Santaballa JA. Aqueous chemistry of N-halo-compounds. Chem Soc Rev. 1998;27:453–460. [Google Scholar]
- 149.Hazen SL, Gaut JP, Hsu FF, Crowley JR, d’Avignon A, Heinecke JW. p-Hydroxyphenylacetaldehyde, the major product of L-tyrosine oxidation by the myeloperoxidase-H2O2-chloride system of phagocytes, covalently modifies epsilon-amino groups of protein lysine residues. J Biol Chem. 1997;272:16990–16998. doi: 10.1074/jbc.272.27.16990. [DOI] [PubMed] [Google Scholar]
- 150.Stelmaszynska T, Zgliczynski JM. N-(2-Oxoacyl)amino acids and nitriles as final products of dipeptide chlorination mediated by the myeloperoxidase/H2O2/Cl− system. Eur J Biochem. 1978;92:301–308. doi: 10.1111/j.1432-1033.1978.tb12748.x. [DOI] [PubMed] [Google Scholar]
- 151.Hawkins CL, Davies MJ. The role of aromatic amino acid oxidation, protein unfolding, and aggregation in the hypobromous acid-induced inactivation of trypsin inhibitor and lysozyme. Chem Res Toxicol. 2005;18:1669–1677. doi: 10.1021/tx0502084. [DOI] [PubMed] [Google Scholar]
- 152.Senthilmohan R, Kettle AJ. Bromination and chlorination reactions of myeloperoxidase at physiological concentrations of bromide and chloride. Arch Biochem Biophys. 2006;445:235–244. doi: 10.1016/j.abb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 153.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–969. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 154.Rees MD, Davies MJ. Heparan sulfate degradation via reductive homolysis of its N-Chloro derivatives. J Am Chem Soc. 2006;128:3085–3097. doi: 10.1021/ja0577239. [DOI] [PubMed] [Google Scholar]
- 155.Rees MD, Hawkins CL, Davies MJ. Hypochlorite and superoxide radicals can act synergistically to induce fragmentation of hyaluronan and chondritin sulphates. Biochem J. 2004;381:175–184. doi: 10.1042/BJ20040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Peskin AV, Winterbourn CC. Kinetics of the reactions of hypochlorous acid and amino acid chloramines with thiols, methionine, and ascorbate. Free Radic Biol Med. 2001;30:572–579. doi: 10.1016/s0891-5849(00)00506-2. [DOI] [PubMed] [Google Scholar]
- 157.Vissers MC, Lee WG, Hampton MB. Regulation of apoptosis by vitamin C. Specific protection of the apoptotic machinery against exposure to chlorinated oxidants. J Biol Chem. 2001;276:46835–46840. doi: 10.1074/jbc.M107664200. [DOI] [PubMed] [Google Scholar]
- 158.Test ST, Lampert MB, Ossanna PJ, Thoene JG, Weiss SJ. Generation of nitrogen-chlorine oxidants by human phagocytes. J Clin Invest. 1984;74:1341–1349. doi: 10.1172/JCI111544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Winterbourn CC, Pichorner H, Kettle AJ. Myeloperoxidase-dependent generation of a tyrosine peroxide by neutrophils. Arch Biochem Biophys. 1997;338:15–21. doi: 10.1006/abbi.1996.9773. [DOI] [PubMed] [Google Scholar]
- 160.Winterbourn CC, Parsons-Mair HN, Gebicki S, Gebicki JM, Davies MJ. Requirements for superoxide-dependent tyrosine hydroperoxide formation in peptides. Biochem J. 2004;381:241–248. doi: 10.1042/BJ20040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Das AB, Nagy P, Abbott HF, Winterbourn CC, Kettle AJ. Reactions of superoxide with the myoglobin tyrosyl radical. Free Radic Biol Med. 2010;48:1540–1547. doi: 10.1016/j.freeradbiomed.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 162.Nagy P, Kettle AJ, Winterbourn CC. Superoxide-mediated formation of tyrosine hydroperoxides and methionine sulfoxide in peptides through radical addition and intramolecular oxygen transfer. J Biol Chem. 2009;284:14723–14733. doi: 10.1074/jbc.M809396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nagy P, Kettle AJ, Winterbourn CC. Neutrophil-mediated oxidation of enkephalins via myeloperoxidase-dependent addition of superoxide. Free Radic Biol Med. 2010;49:792–799. doi: 10.1016/j.freeradbiomed.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 164.Ximenes VF, Silva SO, Rodrigues MR, et al. Superoxide-dependent oxidation of melatonin by myeloperoxidase. J Biol Chem. 2005;280:38160–38169. doi: 10.1074/jbc.M506384200. [DOI] [PubMed] [Google Scholar]
- 165.Ximenes VF, Maghzal GJ, Turner R, Kato Y, Winterbourn CC, Kettle AJ. Serotonin as a physiological substrate for myeloperoxidase and its superoxide-dependent oxidation to cytotoxic tryptamine-4,5-dione. Biochem J. 2009;425:285–293. doi: 10.1042/BJ20090776. [DOI] [PubMed] [Google Scholar]
- 166.Davies MJ, Fu S, Dean RT. Protein hydroperoxides can give rise to reactive free radicals. Biochem J. 1995;305:643–649. doi: 10.1042/bj3050643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochem Photobiol Sci. 2004;3:17–25. doi: 10.1039/b307576c. [DOI] [PubMed] [Google Scholar]
- 168.Davies MJ. The oxidative environment and protein damage. Biochim Biophys Acta. 2005;1703:93–109. doi: 10.1016/j.bbapap.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 169.Rahmanto AS, Morgan PE, Hawkins CL, Davies MJ. Cellular effects of peptide and protein hydroperoxides. Free Radic Biol Med. 2010;48:1071–1078. doi: 10.1016/j.freeradbiomed.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 170.Zgliczyński JM, Stelmaszyńska T, Domański J, Ostrowski W. Chloramines as intermediates of oxidation reaction of amino acids by myeloperoxidase. Biochim Biophys Acta. 1971;235:419–424. doi: 10.1016/0005-2744(71)90281-6. [DOI] [PubMed] [Google Scholar]
- 171.Hazen SL, d’Avignon A, Anderson MM, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to oxidise α-amino-acids to a family of reactive aldehydes. J Biol Chem. 1998;273:4997–5005. doi: 10.1074/jbc.273.9.4997. [DOI] [PubMed] [Google Scholar]
- 172.Hazen SL, Gaut JP, Crowley JR, Hsu FF, Heinecke JW. Elevated levels of protein-bound p-hydroxyphenylacetaldehyde, an amino- acid-derived aldehyde generated by myeloperoxidase, are present in human fatty streaks, intermediate lesions and advanced atherosclerotic lesions. Biochem J. 2000;352:693–699. [PMC free article] [PubMed] [Google Scholar]
- 173.Baynes JW. The Maillard hypothesis on aging: time to focus on DNA. Ann N Y Acad Sci. 2002;959:360–367. doi: 10.1111/j.1749-6632.2002.tb02107.x. [DOI] [PubMed] [Google Scholar]
- 174.Lentner C, editor. Geigy Scientific Tables vol. 3: Physical Chemistry, Composition of Blood, Hematology, Somatometric Data. Basle: Ciba-Geigy Ltd; 1984. [Google Scholar]
- 175.Arlandson M, Decker T, Roongta VA, et al. Eosinophil peroxidase oxidation of thiocyanate. Characterization of major reaction products and a potential sulfhydryl-targeted cytotoxicity system. J Biol Chem. 2001;276:215–224. doi: 10.1074/jbc.M004881200. [DOI] [PubMed] [Google Scholar]
- 176.Kettle AJ, Winterbourn CC. A kinetic analysis of the catalase activity of myeloperoxidase. Biochemistry. 2001;40:10204–10212. doi: 10.1021/bi010940b. [DOI] [PubMed] [Google Scholar]
- 177.Jantschko W, Furtmüller PG, Zederbauer M, Lanz M, Jakopitsch C, Obinger C. Direct conversion of ferrous myeloperoxidase to compound II by hydrogen peroxide: an anaerobic stopped-flow study. Biochem Biophys Res Commun. 2003;312:292–298. doi: 10.1016/j.bbrc.2003.10.117. [DOI] [PubMed] [Google Scholar]
- 178.Winterbourn CC, Hampton MB, Livesey JH, Kettle AJ. Modeling the reactions of superoxide and myeloperoxidase in the neutrophil phagosome: implications for microbial killing. J Biol Chem. 2006;281:39860–39869. doi: 10.1074/jbc.M605898200. [DOI] [PubMed] [Google Scholar]
- 179.Kettle AJ, Winterbourn CC. Superoxide enhances hypochlorous acid production by stimulated human neutrophils. Biochim Biophys Acta. 1990;1052:379–385. doi: 10.1016/0167-4889(90)90146-5. [DOI] [PubMed] [Google Scholar]
- 180.Kettle AJ, Winterbourn CC. Superoxide modulates the activity of myeloperoxidase and optimizes the production of hypochlorous acid. Biochem J. 1988;252:529–536. doi: 10.1042/bj2520529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kettle AJ, Gedye CA, Winterbourn CC. Superoxide is an antagonist of antiinflammatory drugs that inhibit hypochlorous acid production by myeloperoxidase. Biochem Pharmacol. 1993;45:2003–2010. doi: 10.1016/0006-2952(93)90010-t. [DOI] [PubMed] [Google Scholar]
- 182.Subrahmanyam VV, Kolachana P, Smith MT. Hydroxylation of phenol to hydroquinone catalyzed by a human myeloperoxidase-superoxide complex: possible implications in benzene-induced myelotoxicity. Free Radic Res Commun. 1991;15:285–296. doi: 10.3109/10715769109105224. [DOI] [PubMed] [Google Scholar]
- 183.Kettle AJ, Winterbourn CC. Superoxide-dependent hydroxylation by myeloperoxidase. J Biol Chem. 1994;269:17146–17151. [PubMed] [Google Scholar]
- 184.Abu-Soud HM, Hazen SL. Nitric oxide is a physiological substrate for mammalian peroxidases. J Biol Chem. 2000;275:37524–37532. doi: 10.1074/jbc.275.48.37524. [DOI] [PubMed] [Google Scholar]
- 185.Lakshmi VM, Nauseef WM, Zenser TV. Myeloperoxidase potentiates nitric oxide-mediated nitrosation. J Biol Chem. 2005;280:1746–1753. doi: 10.1074/jbc.M411263200. [DOI] [PubMed] [Google Scholar]
- 186.Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275:5425–5430. doi: 10.1074/jbc.275.8.5425. [DOI] [PubMed] [Google Scholar]
- 187.Galijasevic S, Saed GM, Hazen SL, Abu-Soud HM. Myeloperoxidase metabolizes thiocyanate in a reaction driven by nitric oxide. Biochemistry. 2006;45:1255–1262. doi: 10.1021/bi051438k. [DOI] [PubMed] [Google Scholar]
- 188.Galijasevic S, Proteasa G, Abdulhamid I, Abu-Soud HM. The potential role of nitric oxide in substrate switching in eosinophil peroxidase. Biochemistry. 2007;46:406–415. doi: 10.1021/bi061177u. [DOI] [PubMed] [Google Scholar]
- 189.Eiserich JP, Baldus S, Brennan ML, et al. Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science. 2002;296:2391–2394. doi: 10.1126/science.1106830. [DOI] [PubMed] [Google Scholar]
- 190.Grubina R, Huang Z, Shiva S, et al. Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin. J Biol Chem. 2007;282:12916–12927. doi: 10.1074/jbc.M700546200. [DOI] [PubMed] [Google Scholar]
- 191.Burner U, Furtmüller PG, Kettle AJ, Koppenol WH, Obinger C. Mechanism of reaction of myeloperoxidase with nitrite. J Biol Chem. 2000;275:20597–20601. doi: 10.1074/jbc.M000181200. [DOI] [PubMed] [Google Scholar]
- 192.van Dalen CJ, Winterbourn CC, Senthilmohan R, Kettle AJ. Nitrite as a substrate and inhibitor of myeloperoxidase. Implications for nitration and hypochlorous acid production at sites of inflammation. J Biol Chem. 2000;275:11638–11644. doi: 10.1074/jbc.275.16.11638. [DOI] [PubMed] [Google Scholar]
- 193.Brennan ML, Wu W, Fu X, et al. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 194.Monzani E, Roncone R, Galliano M, Koppenol WH, Casella L. Mechanistic insight into the peroxidase catalyzed nitration of tyrosine derivatives by nitrite and hydrogen peroxide. Eur J Biochem. 2004;271:895–906. doi: 10.1111/j.1432-1033.2004.03992.x. [DOI] [PubMed] [Google Scholar]
- 195.Sampson JB, Rosen H, Beckman JS. Peroxynitrite-dependent tyrosine nitration catalyzed by superoxide dismutase, myeloperoxidase, and horseradish peroxidase. Methods Enzymol. 1996;269:210–218. doi: 10.1016/s0076-6879(96)69023-5. [DOI] [PubMed] [Google Scholar]
- 196.Floris R, Piersma SR, Yang G, Jones P, Wever R. Interaction of myeloperoxidase with peroxynitrite. A comparison with lactoperoxidase, horseradish peroxidase and catalase. Eur J Biochem. 1993;215:767–775. doi: 10.1111/j.1432-1033.1993.tb18091.x. [DOI] [PubMed] [Google Scholar]
- 197.Furtmüller PG, Jantschko W, Zederbauer M, et al. Peroxynitrite efficiently mediates the interconversion of redox intermediates of myeloperoxidase. Biochem Biophys Res Commun. 2005;337:944–954. doi: 10.1016/j.bbrc.2005.09.138. [DOI] [PubMed] [Google Scholar]
- 198.Zhang C, Yang J, Jacobs JD, Jennings LK. Interaction of myeloperoxidase with vascular NAD(P)H oxidase-derived reactive oxygen species in vasculature: implications for vascular diseases. Am J Physiol Heart Circ Physiol. 2003;285:H2563–H2572. doi: 10.1152/ajpheart.00435.2003. [DOI] [PubMed] [Google Scholar]
- 199.Zhang C, Yang J, Jennings LK. Leukocyte-derived myeloperoxidase amplifies high-glucose-induced endothelial dysfunction through interaction with high-glucose-stimulated, vascular non-leukocyte-derived reactive oxygen species. Diabetes. 2004;53:2950–2959. doi: 10.2337/diabetes.53.11.2950. [DOI] [PubMed] [Google Scholar]
- 200.Dusting GJ, Tan CSW, Jiang F, et al. Potent inhibitors of vascular oxidative stress: Specific block of Nox4-type NADPH oxidase for cardiovascular and neurological disease. Acta Pharmacol Sin. 2006;27:3. [Google Scholar]
- 201.Dusting GJ, Selemidis S, Jiang F. Mechanisms for suppressing NADPH oxidase in the vascular wall. Mem Inst Oswaldo Cruz. 2005;100:97–103. doi: 10.1590/s0074-02762005000900016. [DOI] [PubMed] [Google Scholar]
- 202.Hampton MB, Winterbourn CC. Modification of neutrophil oxidant production with diphenyleneiodonium and its effect on bacterial killing. Free Radic Biol Med. 1995;18:633–639. doi: 10.1016/0891-5849(94)00181-i. [DOI] [PubMed] [Google Scholar]
- 203.Ellis JA, Mayer SJ, Jones OT. The effect of the NADPH oxidase inhibitor diphenyleneiodonium on aerobic and anaerobic microbicidal activities of human neutrophils. Biochem J. 1988;251:887–891. doi: 10.1042/bj2510887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li Y, Trush MA. Diphenyleneiodonium, an NAD(P)H oxidase inhibitor, also potently inhibits mitochondrial reactive oxygen species production. Biochem Biophys Res Commun. 1998;253:295–299. doi: 10.1006/bbrc.1998.9729. [DOI] [PubMed] [Google Scholar]
- 205.Selemidis S, Dusting GJ, Peshavariya H, Kemp-Harper BK, Drummond GR. Nitric oxide suppresses NADPH oxidase-dependent superoxide production by S-nitrosylation in human endothelial cells. Cardiovasc Res. 2007;75:349–358. doi: 10.1016/j.cardiores.2007.03.030. [DOI] [PubMed] [Google Scholar]
- 206.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Park YS, Suzuki K, Mumby S, Taniguchi N, Gutteridge JMC. Antioxidant binding of caeruloplasmin to myeloperoxidase: myeloperoxidase is inhibited, but oxidase, peroxidase and immunoreactive properties of caeruloplasmin remain intact. Free Radic Res. 2000;33:261–265. doi: 10.1080/10715760000301421. [DOI] [PubMed] [Google Scholar]
- 208.Griffin SV, Chapman PT, Lianos EA, Lockwood CM. The inhibition of myeloperoxidase by ceruloplasmin can be reversed by anti-myeloperoxidase antibodies. Kidney Int. 1999;55:917–925. doi: 10.1046/j.1523-1755.1999.055003917.x. [DOI] [PubMed] [Google Scholar]
- 209.Sokolov AV, Ageeva KV, Cherkalina OS, et al. Identification and properties of complexes formed by myeloperoxidase with lipoproteins and ceruloplasmin. Chem Phys Lipids. 2010;163:347–355. doi: 10.1016/j.chemphyslip.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 210.Baldus S, Rudolph V, Roiss M, et al. Heparins increase endothelial nitric oxide bioavailability by liberating vessel-immobilized myeloperoxidase. Circulation. 2006;113:1871–1878. doi: 10.1161/CIRCULATIONAHA.105.590083. [DOI] [PubMed] [Google Scholar]
- 211.Kettle AJ, Winterbourn CC. Mechanism of inhibition of myeloperoxidase by anti-inflammatory drugs. Biochem Pharmacol. 1991;41:1485–1492. doi: 10.1016/0006-2952(91)90565-m. [DOI] [PubMed] [Google Scholar]
- 212.Whiteman M, Rose P, Halliwell B. Inhibition of hypochlorous acid-induced oxidative reactions by nitrite: is nitrite an antioxidant? Biochem Biophys Res Commun. 2003;303:1217–1224. doi: 10.1016/s0006-291x(03)00503-5. [DOI] [PubMed] [Google Scholar]
- 213.Graham GG, Day RO, Milligan MK, Ziegler JB, Kettle AJ. Current concepts of the actions of paracetamol (acetaminophen) and NSAIDs. Inflammopharmacology. 1999;7:255–263. doi: 10.1007/s10787-999-0008-x. [DOI] [PubMed] [Google Scholar]
- 214.Jantschko W, Furtmüller PG, Zederbauer M, et al. Exploitation of the unusual thermodynamic properties of human myeloperoxidase in inhibitor design. Biochem Pharmacol. 2005;69:1149–1157. doi: 10.1016/j.bcp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 215.Rees MD, Bottle SE, Fairfull-Smith KE, Malle E, Whitelock JM, Davies MJ. Inhibition of myeloperoxidase-mediated hypochlorous acid production by nitroxides. Biochem J. 2009;421:79–86. doi: 10.1042/BJ20090309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Meotti FC, Senthilmohan R, Harwood DT, Missau FC, Pizzolatti MG, Kettle AJ. Myricitrin as a substrate and inhibitor of myeloperoxidase: implications for the pharmacological effects of flavonoids. Free Radic Biol Med. 2008;44:109–120. doi: 10.1016/j.freeradbiomed.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 217.Loke WM, Proudfoot JM, McKinley AJ, et al. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J Agric Food Chem. 2008;56:3609–3615. doi: 10.1021/jf8003042. [DOI] [PubMed] [Google Scholar]
- 218.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–1043. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 219.Kettle AJ, Candaeis LP. Oxidation of tryptophan by redox intermediates of myeloperoxidase and inhibition of hypochlorous acid production. Redox Rep. 2000;5:179–184. doi: 10.1179/135100000101535726. [DOI] [PubMed] [Google Scholar]
- 220.Galijasevic S, Abdulhamid I, Abu-Soud HM. Potential role of tryptophan and chloride in the inhibition of human myeloperoxidase. Free Radic Biol Med. 2008;44:1570–1577. doi: 10.1016/j.freeradbiomed.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ximenes VF, Paino IM, Faria-Oliveira OM, Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res. 2005;38:1575–1583. doi: 10.1590/s0100-879x2005001100003. [DOI] [PubMed] [Google Scholar]
- 222.Sliskovic I, Abdulhamid I, Sharma M, Abu-Soud HM. Analysis of the mechanism by which tryptophan analogs inhibit human myeloperoxidase. Free Radic Biol Med. 2009;47:1005–1013. doi: 10.1016/j.freeradbiomed.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 223.Mitchell JB, Xavier S, DeLuca AM, et al. A low molecular weight antioxidant decreases weight and lowers tumour incidence. Free Radic Biol Med. 2003;34:93–102. doi: 10.1016/s0891-5849(02)01193-0. [DOI] [PubMed] [Google Scholar]
- 224.Burner U, Obinger C, Paumann M, Furtmüller PG, Kettle AJ. Transient and steady-state kinetics of the oxidation of substituted benzoic acid hydrazides by myeloperoxidase. J Biol Chem. 1999;274:9494–9502. doi: 10.1074/jbc.274.14.9494. [DOI] [PubMed] [Google Scholar]
- 225.Kettle AJ, Gedye CA, Hampton MB, Winterbourn CC. Inhibition of myeloperoxidase by benzoic acid hydrazides. Biochem J. 1995;308:559–563. doi: 10.1042/bj3080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Kettle AJ, Gedye CA, Winterbourn CC. Mechanism of inactivation of myeloperoxidase by 4-aminobenzoic acid hydrazide. Biochem J. 1997;321:503–508. doi: 10.1042/bj3210503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.van Zyl, Basson K, Uebel RA, van der Walt BJ. Isoniazid-mediated irreversible inhibition of the myeloperoxidase antimicrobial system of the human neutrophil and the effect of thyronines. Biochem Pharmacol. 1989;38:2363–2373. doi: 10.1016/0006-2952(89)90477-2. [DOI] [PubMed] [Google Scholar]