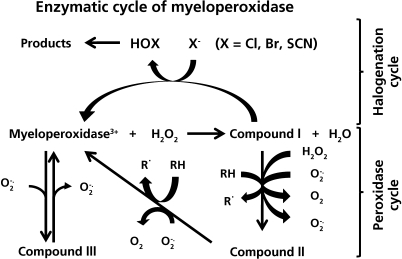
Fig. 1.
The enzymatic cycles of myeloperoxidase. Initial oxidation of the resting iron (III) form of the enzyme by hydrogen peroxide gives rise to Compound I, which is formally an iron (V) species. This intermediate can then undergo either two electron reduction with halide or pseudohalide ions to form hypohalous acids (the halogenation cycle) or undergo two successive one-electron reductions, via Compound II, with consequent radical formation (the peroxidase cycle). The iron (III) form of the enzyme can alos undergo one electron reduction with superoxide radicals to give Compound III. This latter reaction accounts for the SOD mimetic activity of myeloperoxidase.