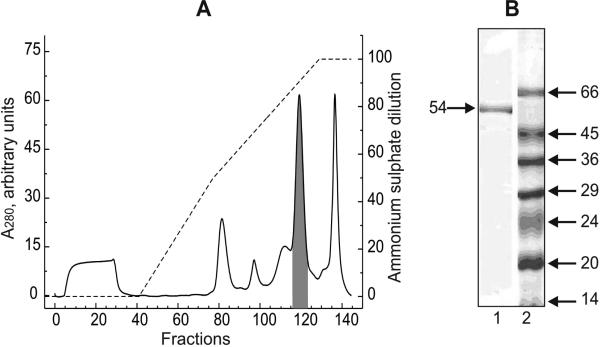
Fig. 1.
A, Chromatography of Q-Sepharose pooled fractions on Phenyl-Sepharose. The details of the chromatography are described in Materials and Methods section. Solid line, protein elution profile; broken line, ammonium sulfate descending gradient. Shadowed peak corresponds to the fractions showing NADH-supported hydrogen peroxide generation and NADH: lipoamide oxidoreductase activities. Seventy one and eighty one per cent of the total protein applied on the column NADH-supported H2O2 production and lipoamide reduction activities, respectively, were recovered in the fractions 117-127 (shadowed peak). A sample of the peak fraction was diluted 1.5-fold by denaturating solution and 5 μl of the mixture were applied on the gel. B, Lane 1, SDS-PAGE of the protein showing hydrogen peroxide generating and lipoamide reductase activities. Lane 2, molecular mass markers. The apparent molecular mass (kDa) is shown on the right.