Abstract
Naked mole-rats are eusocial rodents that live in large social groups with a strict reproductive hierarchy. In each colony only a few individuals breed; all others are non-reproductive subordinates. We previously showed that breeders have increased volume of several brain regions linked to reproduction: the paraventricular nucleus of the hypothalamus (PVN), the principal nucleus of the bed nucleus of the stria terminalis (BSTp), and the medial amygdala (MeA). Breeders also have more large motoneurons in Onuf’s nucleus (ON) in the spinal cord, a cell group innervating perineal muscles that attach to the genitalia. Here, we sought to determine triggers for the neural changes seen in breeders. Specifically, we compared four groups of animals: subordinates, paired animals that did not reproduce, gonadally intact breeders, and gonadectomized breeders. We find that pairing alone is sufficient to cause breeder-like changes in volume of the PVN and cell size distribution in ON. In contrast, increases in BSTp volume were seen only in animals that actually reproduced. Those changes that were seen in successful breeders appear to be independent of gonadal steroids because long-term gonadectomy did not reverse the breeder-like neural changes in the PVN, BSTp or ON, although a trend for gonadectomized animals having larger MeA volumes was detected. Thus, neural changes associated with breeding status in naked mole-rats may be triggered by different aspects of the social and reproductive environment; once changes occur they are largely independent of gonadal hormones and may be permanent.
Keywords: bed nucleus of the stria terminalis, naked mole-rat, neuroplasticity, Onuf’s nucleus, paraventricular nucleus, social status
1. Introduction
Social interactions are important regulators of reproduction in highly social mammals. This is most strikingly seen in cooperatively breeding species in which many or most individuals at least temporarily forego direct reproductive efforts in order to facilitate those of socially dominant conspecifics. For example, puberty is delayed in females of some callitrichid primate species when they remain within their family group [1] and reproduction is actively suppressed in post-pubertal subordinate wolves and mongooses [2, 3]. While great strides have been made in understanding the endocrine correlates of cooperative breeding and reproductive suppression, much less is known about the role of the central nervous system, i.e., how the brain controls the expression of cooperative breeding and related social behaviors or how these complex social interactions may in turn influence the brain.
Naked mole-rats are a powerful animal model in which to study the effects of social status on development and plasticity of the mammalian nervous system. These small rodents can be raised successfully in the laboratory and are one of very few mammalian species that exhibit eusociality [4], an extreme form of cooperative breeding in which reproductive skew is both very high and very stable. Naked mole-rats live in large colonies of up to 300 individuals and reproduction is restricted to a single breeding female and one to three breeding males [5, 6]. Breeders of both sexes are socially dominant over all other members of the colony; the remaining animals in the colony, called subordinates, are non-reproductive. The prevailing view is that dominance behaviors by the queen suppress reproduction in subordinates [7, 8], although other factors cannot be ruled out.
Several behavioral variables differ between breeders and subordinates. While subordinates participate in pup tending, only breeders exhibit direct reproductive behaviors, including copulation and nursing [9, 10]. In addition, mutual genital nuzzling is an affiliative behavior that is almost exclusively displayed by the breeders in a colony [9-11]. Significant endocrine differences also exist between breeders and subordinates. Urinary and plasma progesterone levels are lower in subordinate than in breeding females [12, 13; MM Holmes and MB Lovern, unpublished] and the ovaries are undeveloped and anovulatory in subordinates [9]. While sperm are present in the reproductive tract of subordinate males, there are fewer of them and they are less motile than those of breeding males [14]. Urinary and plasma testosterone also is lower in subordinates than breeders of both sexes [15, 16; Holmes and Lovern, unpublished).
We recently found that status differences extend to the nervous systems of these animals. The paraventricular nucleus of the hypothalamus (PVN), principal nucleus of the bed nucleus of the stria terminalis (BSTp), and the medial amygdala (MeA) were all larger in volume in breeders than subordinates [17]. Breeders also had more large motoneurons in Onuf’s nucleus (ON) in the lumbosacral spinal cord, as well as increases in the size of two target muscles of these motoneurons, which attach to the genitalia [18]. These changes might contribute to the differences in behavior and neuroendocrine function seen between subordinates and breeders.
It is not known what triggers the neural changes previously seen in breeders. When a subordinate becomes a breeder, it experiences an altered endocrine as well as social milieu. Experiential factors such as sexual behavior and/or the production of offspring could also contribute to neural changes. In our laboratory, we create new colonies by removing subordinates from their natal colony and pairing them with opposite sex mates. Many of these pairs successfully produce and rear offspring to establish new colonies. Other pairs produce one or more litters but do not raise them. Still others never produce a litter. Here, we took advantage of this variability to address several questions related to the social and hormonal factors that cause the neural differences previously seen in subordinate and breeding animals.
It is possible, for example, that simply removing animals from their natal colonies and/or pairing them with another animal might be sufficient to induce breeder-like neural changes. If so, then increased volume in the PVN, BSTp or MeA, or altered cell size distribution in ON, might be seen in paired animals, whether or not litters were produced. Alternatively, the production of offspring might be a critical trigger; this could be tested by comparing animals that never produced a litter to those that had. Finally, as mentioned above, increases in gonadal hormones are seen when an animal becomes a breeder [12, 15, 16, 19, 20] and these endocrine changes might be required to maintain a breeder-like nervous system. To test this, we compared gonadally intact and gonadectomized breeders.
2. Material and Methods
2.1 Animals and Tissue Collection
All procedures adhered to institutional and federal animal care and use guidelines. General housing conditions and details of animal care have been described previously [18]. Naked mole-rats reach adult body size anywhere between 4 and 24 months of age and they are very long-lived, surviving for over 20 years in captivity [21, 22]. The current experiment compared four groups: 1) subordinates (N=4 males and 5 females); 2) paired animals (N=6 for each sex); 3) gonadally intact breeders (N=6 for each sex), and 4) gonadectomized (GDX) breeders (N=3 for each sex). Subordinates were animals that remained in their natal colonies until tissue collection (3-6 years old). Paired animals (2-11 years old) were subordinates that were removed from their natal colonies and paired with an opposite sex mate for at least 15 months but had never produced a litter. Intact breeders (5-6 years old) were animals that were paired for at least 7 months and produced at least one litter, but did not successfully raise any of the litters born. GDX breeders had been gonadectomized at least one year prior to collection and were the same animals used in the behavior study by Goldman et al [11]. They had been paired for at least ten years prior to gonadectomy and had produced and raised at least one litter. All groups of animals were sacrificed during the same collection period.
Due to the scarcity of animals, and the fact that established breeders are crucial to the continuation of our colonies, we were not able to include a group of successful breeders that had produced and raised young, were living in the colony with subordinates, and were still gonadally intact. We recognized that the absence of this group would limit the interpretation of some outcomes of this study (i.e., those in which the dependent measure did not vary significantly across groups), while other conclusions would not be affected. This is discussed further below.
Subordinates, paired animals, and intact breeders did not differ significantly in mean age, although GDX breeders were significantly older than all other groups (p < 0.05). Paired animals and intact breeders did not differ in the amount of time paired, while GDX breeders were paired longer than the other groups (p <0.05).
Animals were anesthetized with avertin, rapidly decapitated, and trunk blood was collected for future analyses. Brains were removed, immersion fixed in 5% acrolein in phosphate buffer for 4 hours, transferred to 30% sucrose in phosphate buffer and frozen-sectioned at 30μm in the coronal plane, as previously [17]. Alternate sections were mounted onto gelatin-subbed slides and stained with thionin.
For analysis of ON, vertebral columns were dissected out and immersion-fixed in formalin, transferred to Bouin’s solution and embedded in paraffin. Lumbosacral spinal cords were coronally sectioned at 10μm, mounted on gelatin-subbed slides and stained with Klüver-Barrera, as previously [18]. We previously found that when an animal becomes a breeder, the number of large motoneurons in ON increased while at the same time, a population of smaller cells present in the nucleus decreased [18]. These “small cells” did not meet the normal criteria for motoneurons; in addition to being smaller, the prominent nucleoli that characterize large motoneurons were not detectable in most cases. In the current study, staining was optimized, allowing us to detect nucleoli in even the smallest of cells. “Small cells” and “large cells” in ON were therefore classified strictly based on soma size in the current study, as described below.
2.2 Brain and Spinal Cord Analyses
Analyses of the three brain regions (PVN, BSTp, and MeA) were performed using StereoInvestigator software (MicroBrightfield, Williston VT), as previously [17]. To calculate regional volume, outlines of each region were traced through its rostral-caudal extent, and the summed areas multiplied by section thickness. Reported volume data are unilateral estimates. Potential laterality effects were not addressed as we were unable to reliably distinguish left versus right hemispheres for all animals.
In Klüver-Barrera stained spinal cord sections, ON stands out as a myelin-poor region surrounded by a halo of fibers. All cells within the halo that contained visible nucleoli were traced in 25 sections through ON of each animal and the cross-sectional area determined using StereoInvestigator software. Cell sizes in the current study were not directly comparable to those of Seney et al. [18] because of longer fixation times and greater tissue shrinkage in the previous study. A frequency distribution of the soma size of all ON cells revealed a cluster of cells in subordinates that ranged between 200 and 375 μm2, with a peak frequency of approximately 275 μm2 (M. Seney, unpublished). We therefore defined small cells as 200-375 μm2 and large motoneurons as greater than 375 μm2 (largest soma sizes in ON exceeded 1,000 μm2). To test for the shift in cell size distribution seen previously, the number of large and small cells was counted and percentage of large cells in ON computed for each animal. As a control, a similar cell size analysis was performed for the retrodorsolateral nucleus (RDLN), a motor pool in the lateral motor column at the same level as ON, which innervates muscles of the hind foot in other rodents [23].
All measures were performed on slides coded to conceal the sex and group of the animals. Tissue artifact caused some animals to be removed from analyses. Final number of animals per group for each measure are presented in the Figures.
2.3 Statistical Analyses
As in our earlier reports [17, 18], there was no effect of sex in any of the regions examined. Thus, data from males and females were combined in all analyses. For each dependent measure we performed an overall one-way ANOVA including all groups and then tested three specific hypotheses using planned contrasts. Hypothesis 1, that pairing is sufficient to induce the breeder-like increases in brain volume and ON cell size seen previously, was tested by comparing subordinates with paired animals that had never produced a litter. Hypothesis 2 was that reproduction (production of a litter) is required for the increases in brain volume and ON cell size seen previously. This hypothesis was tested by contrasting animals that had not produced offspring (subordinates and paired animals) to gonadally intact breeders. For any dependent variable in which Hypothesis 1 was supported (i.e., pairing caused a significant change) we also asked if production of offspring caused an additional significant increase by comparing paired animals to gonadally intact breeders. Hypothesis 3 tested whether the gonads are required to maintain breeder-like changes in the nervous system by comparing gonadally intact vs. GDX breeders.
3. Results
As expected from previous results [17, 18], no sex differences were detected on any measure. Data from males and females were therefore combined in all analyses reported below. One-way ANOVA revealed a significant effect of group on volume of the PVN and percent of large cells in ON (F3,32 = 3.45; p = 0.03 and F3,33 = 3.72; p = 0.02, respectively). Planned contrasts indicated that paired animals had significantly larger PVN volume (p = 0.02; Figure 1A) and greater percent of large cells in ON (p = 0.02; Figure 2A) than did subordinates. Producing a litter did not further increase PVN volume or ON cell size over that in paired animals (p = 0.60 and p = 0.94, respectively). Moreover, gonadectomy of breeders did not reverse the increases in PVN volume or percent of large cells in ON; GDX breeders did not differ from intact breeders on either measure (p = 0.25 and p = 0.37, respectively). If anything, the percent of large cells in ON was (non-significantly) larger in GDX compared to intact breeders. In the RDLN, a control spinal nucleus, the majority of cells in all groups were large motoneurons and neither the overall ANOVA (p = 0.23) nor any of the planned contrasts were significant (Figure 2B). Taken together, Hypothesis 1 was supported for PVN volume and ON cell size distribution, suggesting that removal from the colony and pairing with an opposite sex mate is sufficient to induce the neural changes previously seen in breeders. Actual reproduction is not necessary and once the change has occurred, it is not reversed by gonadectomy.
Figure 1.
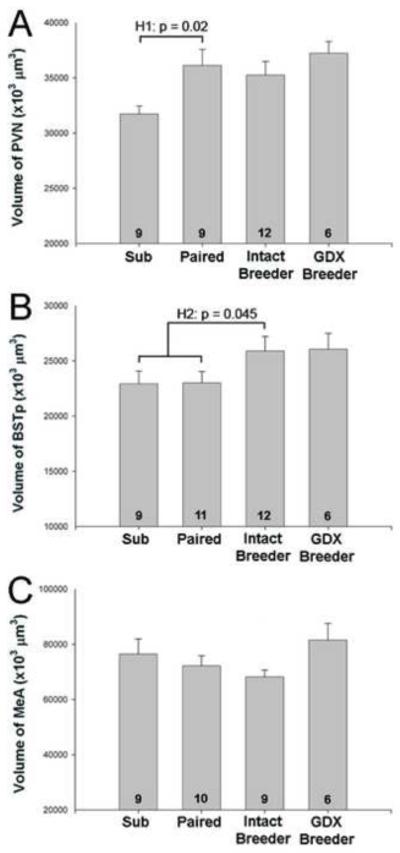
Mean (+ SEM) regional volume of the PVN (A), BSTp (B), and MeA (C) in subordinates (Sub), paired animals that had not produced a litter (Paired), gonadally intact breeders and gonadectomized (GDX) breeders. Data from males and females are combined for each group. Number of animals per group is noted at the base of each bar. H1 indicates statistically significant support for Hypothesis 1 in (A), that removal from the colony and/or pairing is sufficient to induce breeder-like increases in PVN volume. H2 in (B) indicates statistically significant support for Hypothesis 2, that reproduction (production of a litter) is required for increases in BSTp volume.
Figure 2.
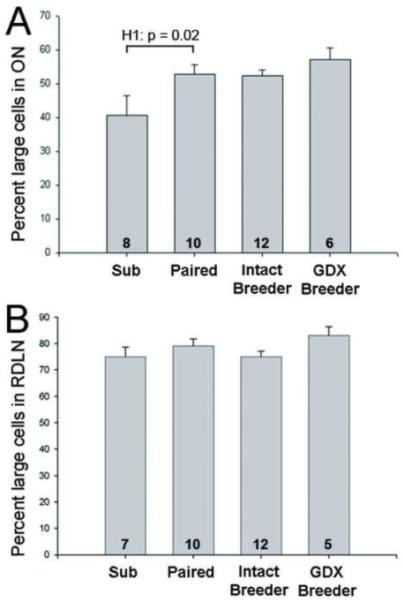
Mean (+ SEM) percentage of large cells in ON (A) and the RDLN (B). Data from males and females are combined for each group. Number of animals per group is noted at the base of each bar. H1 in (A) indicates statistically significant support for Hypothesis 1, that removal from the colony and/or pairing is sufficient to induce breeder-like increases in the percentage of large cells in ON.
The overall ANOVA for BSTp volume did not reach significance (F3,34 = 1.89; p = 0.15) and paired animals did not differ from subordinates (p = 0.97). Thus, pairing was not sufficient to causes changes in the BSTp. Hypothesis 2, however, that reproduction causes the neural increases seen previously, received support. Comparison of intact breeders with animals that had not produced a litter (subordinate and paired groups combined) revealed significantly larger BSTp volumes in animals that had produced a litter (p = 0.045; Figure 1B). Gonadectomy of breeders did not reduce BSTp volume (p = 0.93). Taken together, the data suggest that production of a litter is required for increases in BSTp size seen previously. As with the PVN and ON, gonadectomy of breeders did not reverse the increase.
In the MeA we found no significant main effect of group (F3,30 = 1.50; p = 0.24) and none of the planned contrasts were significant. However, we did note a trend for GDX breeders to have larger MeA volumes than intact breeders (p = 0.06; Figure 1C). The implications of these results are discussed further below.
4. Discussion
Several behavioral, morphological, physiological and neuroanatomical features vary with social status in naked mole-rats [e.g., 9, 14, 17, 18, 24-26]. Previous reports, however, have generally not teased apart the specific factors that cause differences between breeders and subordinates. Here we report that some neural differences between subordinates and breeders are triggered by removing animals from the natal colony and pairing them, whereas other variables seem to require reproductive experience. Yet other neural features are not changed between breeders and subordinates (cortical thickness and volume of the suprachiasmatic nucleus and anterior cortical amygdaloid nucleus in our previous study [17], as well as RDLN measures here), confirming that effects are regionally specific. In this study, as previously, no sex differences and no sex-by-group interactions were seen on any neural measure. Taken together, these findings confirm that reproductive or social status, rather than sex, is predictive of neural morphology and suggest that various components of the change in social and reproductive status are responsible for specific neural differences between subordinates and breeders.
The interpretations of this study have several limitations. In particular, the increase in MeA volume seen previously in breeders [17] was not replicated here. Breeders in the previous study were gonadally intact, had produced and reared offspring, and were living with their offspring at the time of sacrifice. The current study did not include a group matching all of these characteristics, nor were we able to assess potential laterality effects on MeA morphology [27]. Thus, while it is possible that the continued presence of gonadal hormones, dominance interactions and other cues present in a colony setting are all required for an increase in MeA volume, our data for this brain region are inconclusive. It is also possible that we might have seen even larger volume changes in the PVN and BSTp in gonadally intact breeders living with subordinates than we did in our paired and intact breeders here. That seems somewhat unlikely, however, because both the absolute volumes and the magnitude of changes in the PVN and BSTp seen here were very similar to those observed previously [17].
Pairing was sufficient to increase the volume of the PVN and percent of large cells in ON of the spinal cord. Thus, neither reproduction, nor the presence of subordinates in the colony is required for PVN or ON changes. Similarly, vertebral lengthening is observed in female naked mole-rats following pairing and prior to the first pregnancy [28]. It remains to be determined for both neural and skeletal changes exactly which aspects of pairing are important. When an animal is paired, it is removed from colony cues with ensuing social and endocrine changes. Because subordinates never exhibit sexual behaviors within the colony, but do so after pairing, cues that suppress reproduction are likely present in the colony. Several authors have suggested that “shoving” and other dominance behaviors by the queen suppress reproduction in colony subordinates [7, 8]. Olfactory cues could also play a role, although two previous studies have found no evidence for a role for urinary pheromones in reproductive inhibition [8, 29]. Regardless of the nature of the suppressive cue(s), virtually all paired animals, including those in the current study, soon begin mutual genital nuzzling, a vigorous and stereotyped behavior that occurs almost exclusively between breeders in a colony setting. Because the onset of this behavior is rapid (sometimes within minutes of pairing; our own observations), it is unlikely to require changes in neural morphology, but could be a trigger of those changes.
Changes in urinary hormones suggestive of activation of the reproductive axis are also seen soon after a subordinate is removed from its colony. Specifically, increases in progesterone in females and testosterone in males are evident within 1-2 weeks of removal from the colony [e.g., 12, 30], although the first pregnancy after pairing usually requires at least several months. Thus, although the “trigger” for the neural changes observed here is likely to be removal from the colony, the endocrine and behavioral changes that follow could act in concert or sequentially to create the breeder brain. Further distinguishing the relative importance of removal from the colony versus pairing with another animal (e.g., by comparing the brains of single-housed to paired animals) is an important future direction.
Pairing was not sufficient to increase BSTp volume but intact and GDX breeders had larger BSTp volumes than non-reproducing groups, suggesting that this change requires the production of offspring. Because the gonadally intact breeders in our study were not living in a colony setting with subordinates, the confound of “reproduction” with “social dominance” was avoided. Thus, we can conclude that some morphological changes do not require the presence of a colony or the experience of social dominance over others.
Interestingly, none of the breeder-like neural changes were significantly affected by gonadectomy. Each of the neural areas examined express high levels of androgen and/or estrogen receptors in rats and mice [e.g., 31-34] and are hormone-dependent. For example, motoneurons innervating the perineal muscles are highly androgen sensitive in other rodents and decrease to about half their size following gonadectomy of adult males [35-37]. We did not see a decrease in ON soma size after long term GDX in naked mole-rats and, if anything, these animals had the highest percentages of large cells in the study. Similarly, the volume of the MeA (specifically the posterodorsal nucleus) is also reduced after gonadectomy in adulthood in rats [38, 39], yet we report a trend for the MeA to be, if anything, larger in GDX animals. As part of a separate study, gonadal steroid levels were measured in the animals examined here and, as expected, testosterone was markedly reduced in GDX males (Zhou, Holmes, Forger, Goldman, Lovern, Caraty, Faulkes, & Coen, in preparation). Moreover, the MeA, BSTp and PVN all express androgen receptor immunoreactivity in naked mole-rats [25], so the lack of effect of gonadectomy is not due to an absence of steroid receptors in these areas.
However, the GDX breeders in the current study all were gonadectomized after becoming established breeders. Thus, while our data demonstrate that the gonads are not required for the maintenance of changes in the PVN, BSTp and ON, they do not address whether gonadal steroids are required to initiate the effects. In this scenario, hormonal changes seen after pairing may initiate a late “organizational” period, during which irreversible neural changes occur in the nervous systems of future breeders. This is an interesting possibility that could be tested by gonadectomizing subordinates prior to pairing them with an opposite sex individual.
The GDX breeders used in this study also continued to exhibit some behaviors characteristic of breeders. These animals were part of a study in which behavioral observations within the colony were conducted for up to 5 months post-gonadectomy of both members of the breeding pair [11]. Although castration of breeder males eliminated the display of copulatory behavior, GDX breeders continued to exhibit the intense mutual genital nuzzling characteristic of the breeder pair and to inhibit nuzzling in subordinates [11]. In this sense, GDX breeders retain their social status even in the absence of gonadal hormones. Our observation that the neural changes in intact breeders persist following GDX supplies a possible neural correlate.
What the changes in neural volume and ON cell size mean functionally is not known, although all of the regions examined here are related to reproduction. Motoneurons in ON, or in homologous cell groups in other species, control ejaculatory reflexes in males [40] and these motoneurons and the muscles they innervate are often absent or vestigial in females [31]. In naked mole-rats, the motoneurons are not only present in females, but similar in morphology in both sexes; what their function is in females is not clear. The BSTp plays a major role in the processing of pheromonal input in other rodents [41] and has associated roles in the control of male sexual behavior and luteinizing hormone surges. The BSTp also has been implicated in the regulation of stress responses in other rodents via its projections to the PVN [42]. The PVN is involved in both the hypothalamic-pituitary-gonadal and hypothalamic-pituitary-adrenal (HPA) axes and is likely engaged during changes in subordinate/dominant relationships, particularly when they are directly linked to reproduction as in this species. Unfortunately, the relationship between HPA function and social status in naked mole-rats is not yet clear; one study found a significant relationship between glucocorticoids and social status [19] while another did not [16]. It will be interesting to examine phenotypically identified cells to determine if, for example, corticotrophin-releasing hormone cells in the PVN, which control adrenal function, are specifically affected when an animal becomes a breeder. Although much remains to be learned, this study points to specific neural regions and dissociable environmental triggers likely to be involved in plasticity of the social brain.
Research Highlights.
PVN volume increased following removal from the colony and pairing.
Cell size in ON was affected by removal from the colony and pairing.
Increases in BSTp volume were seen only in animals that actually reproduced.
Gonadectomy did not reverse the breeder-like neural changes in the PVN, BSTp or ON.
Table 1.
Results for specific hypothesis testing
|
Hypothesis 1: Removal from colony and/or pairing is sufficient to cause change |
Hypothesis 2: Production of offspring required for change |
Hypothesis 3: Gonads required to maintain change |
|
|---|---|---|---|
| PVN volume | Yes | No | No |
| BSTp volume | No | Yes | No |
| MeA volume | No | ? | ? |
|
ON % large
cells |
Yes | No | No |
Acknowledgements
The authors are grateful to Sharry Goldman for invaluable assistance with animal husbandry. This work was supported by NSF (IOS-0642050 to NGF and BDG), NIMH (K02 MH072825 to NGF), NINDS (F31 NS058258 to MLS) and CIHR (FRN 76508 to MMH).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- [1].French JA. Proximate regulation of singular breeding in callitrichid primates. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; USA: 1997. pp. 34–75. [Google Scholar]
- [2].Asa CS. Hormonal and experiential factors in the expression of social and parental behavior in canids. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; USA: 1997. pp. 129–49. [Google Scholar]
- [3].Creel SR, Waser PM. Variation in reproductive suppression among dwarf mongooses: Interplay between mechanisms and evolution. In: Solomon NG, French JA, editors. Cooperative Breeding in Mammals. Cambridge University Press; USA: 1997. pp. 150–70. [Google Scholar]
- [4].Jarvis JUM. Eusociality in a mammal: cooperative breeding in naked mole-rat colonies. Science. 1981;212:571–3. doi: 10.1126/science.7209555. [DOI] [PubMed] [Google Scholar]
- [5].Brett RA. The population structure of naked mole-rat colonies. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; USA: 1991. pp. 97–136. [Google Scholar]
- [6].Lacey EA, Sherman PW. Social organization of naked mole-rat colonies: Evidence for division of labor. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; USA: 1991. pp. 275–336. [Google Scholar]
- [7].Faulkes CG, Abbott DH. Evidence that primer pheromones do not cause social suppression of reproduction in male and female naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1993;99:225–30. doi: 10.1530/jrf.0.0990225. [DOI] [PubMed] [Google Scholar]
- [8].Reeve HK. Queen activation of lazy workers in colonies of the eusocial naked mole-rat. Nature. 1992;358:147–9. doi: 10.1038/358147a0. [DOI] [PubMed] [Google Scholar]
- [9].Jarvis JUM. Reproduction of naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; USA: 1991. pp. 384–425. [Google Scholar]
- [10].Lacey EA, Alexander RD, Braude SH, Sherman PW, Jarvis JUM. An ethogram for the naked mole-rat: Nonvocal behaviors. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; USA: 1991. pp. 209–42. [Google Scholar]
- [11].Goldman SL, Forger NG, Goldman BD. Influence of gonadal sex hormones on behavioral components of the reproductive hierarchy in naked mole-rats. Horm Behav. 2006;50:77–84. doi: 10.1016/j.yhbeh.2006.01.013. [DOI] [PubMed] [Google Scholar]
- [12].Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of ovarian cyclicity in captive and wild colonies of naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1990;88:559–68. doi: 10.1530/jrf.0.0880559. [DOI] [PubMed] [Google Scholar]
- [13].Faulkes CG, Abbott DH, Liddell CE, George LM, Jarvis JUM. Hormonal and behavioral aspects of reproductive suppression in female naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; USA: 1991a. pp. 426–45. [Google Scholar]
- [14].Faulkes CG, Trowell SN, Jarvis JUM, Bennett NC. Investigation of numbers and motility of spermatozoa in reproductively active and socially suppressed males of two eusocial African mole-rats, the naked mole rat (Heterocephalus glaber) and the Damaraland mole-rat (Cryptomys damarensis) J Reprod Fertil. 1994;100:411–16. doi: 10.1530/jrf.0.1000411. [DOI] [PubMed] [Google Scholar]
- [15].Faulkes CG, Abbott DH, Jarvis JUM. Social suppression of reproduction in male naked mole-rats, Heterocephalus glaber. J Reprod Fertil. 1991b;91:593–604. doi: 10.1530/jrf.0.0910593. [DOI] [PubMed] [Google Scholar]
- [16].Clarke FM, Faulkes CG. Hormonal and behavioural correlates of male dominance and reproductive status in captive colonies of the naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1998;265:1391–99. doi: 10.1098/rspb.1998.0447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Holmes MM, Rosen GJ, Jordan CL, de Vries GJ, Goldman BD, Forger NG. Social control of brain morphology in a eusocial mammal. Proc Natl Acad Sci USA. 2007;104:10548–52. doi: 10.1073/pnas.0610344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seney M, Goldman BD, Forger NG. Breeding status affects motoneuron number and muscle size in naked mole-rats: Recruitment of perineal motoneurons? J Neurobiol. 2006;66:1354–64. doi: 10.1002/neu.20314. [DOI] [PubMed] [Google Scholar]
- [19].Clarke FM, Faulkes CG. Dominance and queen succession in captive colonies of the eusocial naked mole-rat, Heterocephalus glaber. Proc Biol Sci. 1997;264:993–1000. doi: 10.1098/rspb.1997.0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Margulis SW, Saltzman W, Abbott DH. Behavioral and hormonal changes in female naked mole-rats (Heterocephalus glaber) following removal of the breeding female from a colony. Horm Behav. 1995;29:227–47. doi: 10.1006/hbeh.1995.1017. [DOI] [PubMed] [Google Scholar]
- [21].Buffenstein R. The naked mole-rat: A new long-living model for human aging research. J Gerontol: Biol Sci. 2005;60A:1369–77. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- [22].Jarvis JUM, O’Riain MJ, McDaid E. Growth and factors affecting body size in naked mole-rats. In: Sherman PW, Jarvis JUM, Alexander RD, editors. The Biology of the Naked Mole-Rat. Princeton University Press; USA: 1991. pp. 358–83. [Google Scholar]
- [23].Nicolopoulas-Stournaras S, Iles JF. Motor neuron columns in the lumbar spinal cord of the rat. J Comp Neurol. 1983;217:75–85. doi: 10.1002/cne.902170107. [DOI] [PubMed] [Google Scholar]
- [24].Henry EC, Dengler-Crish CM, Catania KC. Growing out of a caste – reproduction and the making of the queen mole-rat. J Exp Biol. 2007;210:261–8. doi: 10.1242/jeb.02631. [DOI] [PubMed] [Google Scholar]
- [25].Holmes MM, Goldman BD, Forger NG. Social status and sex independently influence androgen receptor expression in the eusocial naked mole-rat brain. Horm Behav. 2008;54:278–85. doi: 10.1016/j.yhbeh.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rosen GJ, de Vries GJ, Goldman SL, Goldman BD, Forger NG. Distribution of vasopessin in the brain of the eusocial naked mole-rat. J Comp Neurol. 2008;500:1093–105. doi: 10.1002/cne.21215. [DOI] [PubMed] [Google Scholar]
- [27].Johnson RT, Breedlove SM, Jordan CL. Sex differences and laterality in astrocyte number and complexity in the adult rat medial amygdala. J Comp Neurol. 2008;511:599–609. doi: 10.1002/cne.21859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dengler-Crish CM, Catania KC. Phenotypic plasticity in female naked mole-rats after removal from reproductive suppression. J Exp Biol. 2007;210:4351–8. doi: 10.1242/jeb.009399. [DOI] [PubMed] [Google Scholar]
- [29].Smith TE, Faulkes CG, Abbott DH. Combined olfactory contact with the parent colony and direct contact with nonbreeding animals does not maintain suppression of ovulation in female naked mole-rats (Heterocephalus glaber) Horm Behav. 1997;31:277–88. doi: 10.1006/hbeh.1997.1384. [DOI] [PubMed] [Google Scholar]
- [30].Faulkes CG, Abbott DH. Social control of reproduction in breeding and non-breeding male naked mole-rats (Heterocephalus glaber) J Reprod Fertil. 1991;93:427–35. doi: 10.1530/jrf.0.0930427. [DOI] [PubMed] [Google Scholar]
- [31].Breedlove SM, Arnold AP. Hormone accumulation in a sexually dimorphic motor nucleus of the rat spinal cord. Science. 1980;210:564–6. doi: 10.1126/science.7423210. [DOI] [PubMed] [Google Scholar]
- [32].Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinol. 1998;139:1594–601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- [33].Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinol. 2003;144:2055–67. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- [34].Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- [35].Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen-insensitive rats. Brain Res. 1981;225:297–307. doi: 10.1016/0006-8993(81)90837-4. [DOI] [PubMed] [Google Scholar]
- [36].Forger NG, Fishman RB, Breedlove SM. Differential effects of testosterone metabolites upon the size of sexually dimorphic motoneurons in adulthood. Horm Behav. 1992;26:204–13. doi: 10.1016/0018-506x(92)90042-t. [DOI] [PubMed] [Google Scholar]
- [37].Zuloaga DG, Morris JA, Monks DA, Breedlove SM, Jordan CL. Androgen-sensitivity of somata and dendrites of spinal nucleus of the bulbocavernosus (SNB) motoneurons in male C57BL6J mice. Horm Behav. 2007;51:207–12. doi: 10.1016/j.yhbeh.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Cooke BM, Tabibnia G, Breedlove SM. A brain sexual dimorphism controlled by adult circulating androgens. Proc Natl Acad Sci USA. 1999;96:7538–40. doi: 10.1073/pnas.96.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdale and sexual arousal in male rats. Horm Behav. 2003;43:336–46. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- [40].Hart BL, Melese-D’Hospital PY. Penile mechanisms and the role of the striated penile muscles in penile reflexes. Physiol Behav. 1983;31:807–13. doi: 10.1016/0031-9384(83)90277-9. [DOI] [PubMed] [Google Scholar]
- [41].Simerly RB. Wired for reproduction: organization and development of sexually dimorphic circuits in the mammalian forebrain. Annu Rev Neurosci. 2002;25:507–36. doi: 10.1146/annurev.neuro.25.112701.142745. [DOI] [PubMed] [Google Scholar]
- [42].Choi DC, Furay AR, Evanson NK, Ulrich-Lai YM, Nguyen MM, Ostrander MM, Herman JP. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology. 2008;33:659–69. doi: 10.1016/j.psyneuen.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


