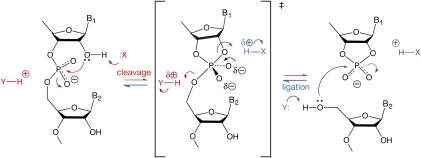
FIGURE 1.
The generally accepted chemical mechanism of cleavage and ligation in the nucleolytic ribozymes, and the possible rate acceleration by general acid–base catalysis. In the cleavage reaction (red) the 2′-O attacks the 3′-P in an SN2 process leading to a trigonal bipyramidal phosphorane that is probably close to the transition state. This is consistent with observed inversion of configuration, although it should be noted that a constrained stepwise mechanism could conceivably result in inversion. Simultaneous breakage of the bond to the 5′-O leads to the cyclic 2′3′ phosphate and 5′-O products. In the ligation reaction (blue), the 5′-O nucleophile attacks the P of the cyclic phosphate. The cleavage reaction can be potentially catalyzed by a general base (X) assisting in the removal of the proton from the 2′-OH, and a general acid (Y) that protonates the 5′-O− oxyanion leaving group. By the principle of microscopic reversibility, X and Y will act as general acid and base, respectively, in the ligation reaction. They have arbitrarily been shown as neutral in the unprotonated states, although this is not necessarily the case.