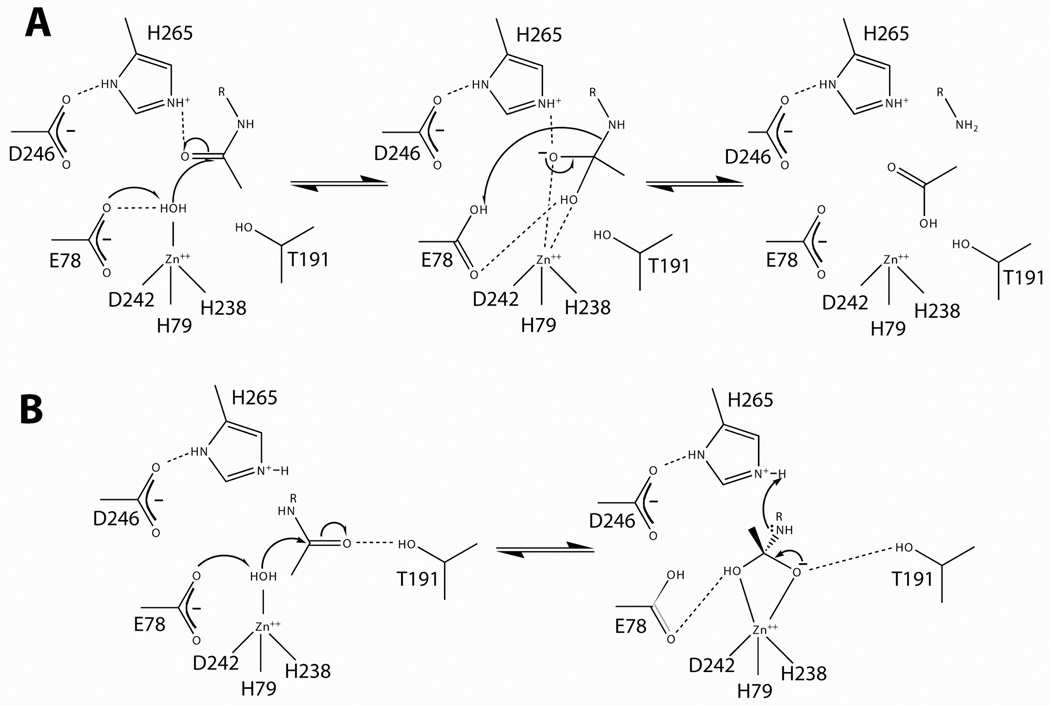
Figure 3. Proposed LpxC mechanisms.
Panel A. The LpxC catalytic mechanism proposed by McClerren and coworkers (2005). This proposal suggests that E78 abstracts a proton from zinc-bound water, thereby activating the water for attack on the carbonyl carbon. The proposal presented here suggests that H265 stabilizes the oxyanion intermediate, and E78 later donates a proton to the terminal amine. Panel B. An alternate hypothesis by Hernick and coworkers (2005) suggests that T191 stabilizes the oxyanion intermediate and H265 donates a proton to the liberated amine.