Abstract
Recovery of photosynthesis in rehydrating desiccated leaves of the poikilochlorophyllous desiccation-tolerant plant Xerophyta scabrida was investigated. Detached leaves were remoistened under 12 h light/dark cycles for 96 h. Water, chlorophyll (Chl), and protein contents, Chl fluorescence, photosynthesis–CO2 concentration response, and the amount and activity of Rubisco were measured at intervals during the rehydration period. Leaf relative water contents reached 87% in 12 h and full turgor in 96 h. Chl synthesis was slower before than after 24 h, and Chla:Chlb ratios changed from 0.13 to 2.6 in 48 h. The maximum quantum efficiency recovered faster during rehydration than the photosystem II operating efficiency and the efficiency factor, which is known to depend mainly on the use of the electron transport chain products. From 24 h to 96 h of rehydration, net carbon fixation was Rubisco limited, rather than electron transport limited. Total Rubisco activity increased during rehydration more than the Rubisco protein content. Desiccated leaves contained, in a close to functional state, more than half the amount of the Rubisco protein present in rehydrated leaves. The results suggest that in X. scabrida leaves Rubisco adopts a special, protective conformation and recovers its activity during rehydration through modifications in redox status.
Keywords: Chlorophyll fluorescence, desiccation tolerance, non-photochemical quenching, photosynthesis, poikilochlorophylly, relative water content, Rubisco, Xerophyta scabrida
Introduction
Desiccation-tolerant (DT) plants can withstand the loss of up to 90–95% of the water of their vegetative tissues and revive when humidity is available, in contrast to the majority of plants (Proctor and Tuba, 2002). Desiccation tolerance entails cellular, biochemical, and molecular changes during dehydration (Vicré et al., 2004), including the accumulation of carbohydrates (Whittaker et al., 2001; Toldi et al., 2009), late embryogenesis-abundant (LEA) proteins (Ingram and Bartels, 1996), and antioxidants (Kranner et al., 2002; Mowla et al., 2002; Vicré et al., 2004), as well as altered expression of target genes and transcription factors (Frank et al., 1998; Ramanjulu and Bartels, 2002). Recovery from the desiccated state is much faster in homoiochlorophyllous DT (HDT) plants such as Haberlea rhodopensis (Georgieva et al., 2005, 2007) than in poikilochlorophyllous DT (PDT) plants such as Xerophyta scabrida (Tuba et al., 1993a, 1994). The former retain their chlorophyll (Chl), preserve their photosynthetic apparatus, and undergo morphological changes during drying that protect their tissues against oxidative stress (Vicré et al., 2004). In contrast, the latter lose all of their Chl and dismantle their photosynthetic apparatus during drying, and they resynthesize these molecules after rehydration (Tuba et al., 1994, 1998a; Sherwin and Farrant, 1996). Xerophyta scabrida preserves most of its Chl when dried in the dark, so most of the loss seems to result from photooxidative degradation (Tuba et al., 1997). The PDT strategy evolved in plants that are anatomically complex and that include the largest in size of all DT species, and it can be seen as the younger strategy in evolutionary terms (Proctor and Tuba, 2002).
DT plants regain their water content within a time span ranging from minutes in bryophytes and pteridophytes (Csintalan et al., 1999) to days in angiosperms (Tuba et al., 1994; Proctor and Tuba, 2002; Georgieva et al., 2005; Degl'Innocenti et al., 2008). As could be expected for HDT plants, the Chl contents, the Chla:Chlb ratio, and the relative amounts of the Chl–protein complexes remain mostly unchanged in control, desiccated, and rehydrated leaves (Georgieva et al., 2005, 2007). In remoistened PDT plants, Chl resynthesis begins after 6–12 h of rehydration (Tuba et al., 1993a, 1994) at 36% relative water contecnt (RWC) (Degl'Innocenti et al., 2008), and is completed by 48–72 h at 84% RWC. Synthesis of the photosystem II (PSII) reaction centre and antenna proteins correlates with the recovery and increase in photosynthetic capacity (Ingle et al., 2008).
Non-radiative energy dissipation can play an important protective role during both desiccation and rehydration. In several mosses (Csintalan et al., 1999) and in Ramonda serbica (Augusti et al., 2001; Degl'Innocenti et al., 2008), non-photochemical quenching (NPQ) shows a transient increase upon remoistening. Maximum quantum efficiency, Fv/Fm, is completely recovered at 48 h (Degl'Innocenti et al., 2008) or 72 h (Tuba et al., 1994) after rewatering. Faster increases during rehydration were recorded in Fv/Fm than in PSII operating efficiency, Fq′/Fm′ (also termed ΦPSII; Csintalan et al., 1999). Nonetheless, the involvement in the non-photochemical energy dissipation of basal, non-radiative decays and of the regulated non-photochemical energy loss (Baker et al., 2007; Klughammer and Schreiber, 2008) during the rehydration of PDT plants has not been investigated.
Full recovery of photochemical activity in PDT plants requires the assimilation of CO2 as an acceptor of the products of photosynthetic electron transport. In the moss Polytrichum formosum, carbon fixation is completely restored 3 h after rewetting (Proctor et al., 2007) but is resumed at 12 h (51% RWC), and is not fully re-established at 48 h (84% RWC) after rehydration in higher plant DT species (Tuba et al., 1994; Degl'Innocenti et al., 2008). Recently, photosynthesis–CO2 concentration responses in hydrated HDT leaves were reported (Peeva and Cornic, 2009), but information concerning the fate of ribulose-1,5-bisphosphate carboxylase oxygenase (Rubisco) and the relative capacities of Rubisco carboxylation and electron transport in rehydrating PDT plants is scarce. In earlier studies, desiccated fronds (Harten and Eickmeier, 1986) and leaves (Daniel and Gaff, 1980) of DT plants conserved from 40% to 62% of the control Rubisco activity. A decrease in Rubisco content was observed during dehydration of the C4 DT plant Sporobolus stapfianus (Martinelli et al., 2007), whereas Rubisco (fraction I) protein did not appear to decrease relative to hydrated X. viscosa leaves (Daniel and Gaff, 1980). Consistent with this, it was surmised that the carboxylating enzymes in X. scabrida would only be inactivated, but not degraded, during desiccation (Tuba et al., 1998b). In contrast, Rubisco activity was undetectable below 51% RWC (12 h rehydration) in R. serbica leaves (Degl'Innocenti et al., 2008). Drying-induced disruption of the electron transport chain causes oxidative stress (Vicré et al., 2004), which can induce aggregation and polymerization, membrane association, and the degradation of Rubisco (Marín-Navarro and Moreno, 2006). On the other hand, in stressed Lemna minor fronds Rubisco was not degraded but gradually became polymerized to inactive aggregates, accompanied by a reduction in the number of sulphydryl groups (Ferreira and Shaw, 1989). The Benson–Calvin cycle enzymes have a tendency to form soluble and membrane-bound multienzyme complexes (Sainis and Harris, 1986; Gontero et al., 1988, 1993; Sainis et al., 1989; Persson and Johansson, 1989; Hermoso et al., 1992; Anderson et al., 1995; Agarwal et al., 2009) with higher catalytic efficiency and less susceptibility to auto-oxidation and proteolysis than free enzymes (Gontero et al., 1988, 1993).
The aim of this study was to determine to what extent the recovery from desiccation of X. scabrida photosynthesis is dependent on photochemical and carboxylation capacities. The hypothesis was that restoration of Rubisco activity limits the attainment of photosynthetic competence of rehydrated PDT plants. To test this hypothesis, Chl fluorescence and photosynthesis–CO2 response curves were determined while turgor was being regained. To assess the carboxylation capacity, the free or aggregated state, as well as the amount and activity of Rubisco were determined in desiccated and rehydrating leaves.
Materials and methods
A description of X. scabrida morphology has been provided in an earlier article (Tuba et al., 1993b). Briefly, it is a 40–90 cm high, branched pseudoshrub with perennial leaves. Dry leaves are usually 24–30 cm long, 5–6 mm wide, and folded over along the midrib. In July 2004, desiccated X. scabrida (Pax) Th. Dur. et Schinz branches were collected in Tanzania (Mindu Hill, WSW of Morogoro town, 6º50.78'S, 37º36.76'E) and were kept in paper bags at room temperature until rehydration and analysis. Central sections of desiccated leaves having a purple-black or blackish-green coloration were selected for this study. As representative of time zero inmediately prior to watering, triplicate desiccated leaves were briefly immersed in water in a vacuum desiccator to saturate the intercellular air spaces with water. Subsequently the leaf material was blotted, weighed, frozen in liquid nitrogen, and stored at –80 °C for Chl, protein, and Rubisco activity measurements (see below). Previous experience (Tuba et al., 1993b) has shown that placing whole plants with their roots in water does not result in a recovery response in X. scabrida, because the roots are dry and unable to transport water to the leaves; only a direct rewatering of the leaves by immersion in water led to regreening. Moreover, in the natural habitat, new root development and water uptake were preceded by the rehydration and regreening of the leaves. Consequently, in the present experiments, additional leaf samples were rehydrated by submerging them in a 10.0 l glass tank filled with tap water and aerated with a pump (Tuba et al., 1993a, 1994). The container was placed in a growth chamber with a 21 °C/15 °C day/night temperature, under a 340 μmol m−2 s−1 photon flux density in a 12 h photoperiod (modified after Tuba et al., 1994). The water was changed daily.
Water contents
Triplicate samples of desiccated leaves were weighed before and after drying in an oven for 48 h at 60 °C; the second of these recordings was taken as the dry weight. This was preferred to the oven-dry weight after full rehydration, which may be affected by losses during rewatering due to respiration or release of soluble compounds. Additional leaves (in triplicate) that were submerged in water were blotted and their fresh weight was determined at 12, 24, 48, 72, 96, and 120 h after the start of rehydration. No further weight gain was recorded after 96 h and this was considered as the turgid weight. The RWCs at successive times in the rehydration period were determined as (fresh weight–dry weight)×100/(turgid weight–dry weight). Chl and protein contents, and Rubisco activity were determined in other leaves sequentially sampled during rehydration (see below) and were expressed on a turgid weight basis. The latter was estimated from the fresh weight of these leaf samples and the water contents measured in the samples used for RWC measurements.
Chl fluorescence and gas exchange measurements
For Chl fluorescence and CO2 assimilation measurements, triplicate leaf samples kept in water were collected between 3 h and 8 h after the start of the photoperiod at the times indicated above and placed in the fluorometer leaf clip or the infrared gas analyser (IRGA) leaf chamber (see below) with both ends of the leaves wrapped in moistened filter paper. After measurements, the leaf samples were returned to the water container in the growth room for a 30 min adaptation period prior to harvesting for leaf analyses (see below). Chl fluorescence was measured with a modulated fluorometer (PAM-2000, Walz, Effeltrich, Germany). Leaf sections were kept in the dark for 20 min with leaf clips (Gutiérrez et al., 2009), after which dark-adapted state fluorescence parameters were measured. Fo was recorded and a saturating flash of light (∼8000 μmol m−2 s−1) was applied for 0.8 s to determine Fm. Fo and Fm, respectively, represent the minimal and maximal fluorescence in the dark-adapted state, and Fv/Fm [(Fm–Fo)/Fm] represents the maximum quantum efficiency. Light-adapted leaves were illuminated with the red actinic light source of the fluorometer to obtain an irradiance of 1500 μmol m−2 s−1. Saturating light pulses were given every 20 s until steady-state Chl fluorescence parameter values were obtained, the fluorescence values being recorded immediately before (F′, steady-state fluorescence) and after (Fm′, maximal fluorescence in the light) each pulse. Then, the leaf was covered with a black cloth, the actinic light was switched off, and an infrared light was switched on for 3 s to quickly reoxidize the PSII centres and measure Fo′, the minimal fluorescence with an NPQ similar to that found in the steady-state under light. The equipment determines Fq′/Fm′ [(Fm′–F′)/Fm′], which is the PSII operating efficiency (also termed ΦPSII) (Baker et al., 2007). The PSII efficiency factor Fq′/Fv′ (also termed qP) [(Fm′–F′)/ (Fm′–Fo′)] and Fv′/Fm′ [(Fm′–Fo′)/Fm′], the PSII maximum efficiency under light, were calculated. The fraction of PSII centres in the open state, qL, equates to (Fq′/Fv′) (Fo′/F′). The quantum yield of basal, non-radiative decays, ΦNO, is 1/[NPQ+1+qL(Fm/Fo–1)], where NPQ is (Fm/Fm′)–1, and the quantum yield of non-photochemical quenching, ΦNPQ, is 1–(Fq′/Fm′)–ΦNO (Kramer et al., 2004).
Light-saturated photosynthesis–CO2 response curves of leaves were recorded at the same times and with the same sampling scheme as Chl fluorescence. Measurements were carried out with an air flow rate of 300 ml min−1, 1500 μmol m−2 s−1 irradiance, and a 1.6±0.23 kPa vapour pressure deficit, using a 1.7 cm2 window leaf chamber connected to a portable IRGA (CIRAS-2, PP Systems, Hitchin, Herts, UK) with differential operation in an open system. Temperature was kept at 25 °C with the Peltier system of the IRGA. The air CO2 concentration was decreased in four steps from 34 Pa to 6 Pa and then increased from 34 Pa to 180 Pa in six steps. Chloroplast CO2 concentration, Cc, the maximum carboxylation rate allowed by Rubisco, Vcmax, and the rate of photosynthetic electron transport, J, were determined from the photosynthesis responses to CO2 with the Rubisco kinetic parameters and the Excel utility of Sharkey et al. (2007).
Rubisco activity assay
Triplicate leaf samples that had been equilibrated in aerated water in the growth chamber after Chl fluorescence and gas exchange measurements were blotted dry, rapidly transferred in situ to liquid nitrogen, and stored at –80 °C until analysed. Rubisco activity was assayed on the basis of the procedure described by Lilley and Walker (1974), modified by Ward and Keys (1989) and Sharkey et al. (1991). Aliquots (80 mg) of frozen leaves were ground in a mortar with liquid nitrogen, extracted with 4 ml of 100 mM N,N-bis(2-hydroxyethyl)glycine (Bicine)-NaOH (pH 7.8), 10 mM MgCl2, 0.5 mM dithiothreitol (DTT), 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol tetraacetic acid (EGTA), 1% (v/v) Triton X-100, 0.25% (w/v) bovine serum albumin (BSA), 20% (v/v) glycerol, 1 mM benzamidine, 1 mM ε-aminocaproic acid, 10 μM leupeptin, and 1 mM phenylmethylsulphonyl fluoride (PMSF), and then centrifuged at 13 000 g. The total time from extraction to the assay of initial Rubisco activity was <2.5 min. Activity was assayed by adding extract (40 μl) to a mixture of 100 mM Bicine (pH 8.2), 20 mM MgCl2, 10 mM NaHCO3, 18 mM KCl, 0.6 mM ribulose-1,5-bisphosphate (RuBP), 0.2 mM NADH, 1 mM ATP, 5 mM creatine phosphate, 25 U ml−1 phosphocreatine kinase, 47U ml−1 phosphoglycerate kinase, 47 U ml−1 glyceraldehyde 3-phosphate dehydrogenase, 10 mM DTT, 1 mM EDTA, 0.02% (w/v) BSA (800 μl total volume) and recording the decrease in absorbance at 340 nm minus 400 nm for 40–60 s, at a stoichiometry of 2:1 between NADH oxidation and RuBP carboxylation. The spectrophotometer cell compartment was thermostated with a circulating water bath. To assay total Rubisco activity, an aliquot of the extract was incubated with NaHCO3 and MgCl2 for 10 min at room temperature before the addition of coupling enzymes and NADH; the reaction was started by adding RuBP. The activation state was estimated as initial activity, as a percentage of total activity. Activation and assays were performed either at room temperature or at 35 °C. Commercial coupling enzymes suspended in ammonium sulphate were precipitated by centrifugation and dissolved in 20% glycerol (Sharkey et al., 1991). With the assay buffer described, the initial lag in the reaction reported by others (Ward and Keys, 1989; Sharkey et al., 1991) was not observed.
Chlorophyll and protein analysis
Total Chl, Chla, and Chlb in 80% acetone extracts of frozen triplicate subsamples were determined according to Arnon (1949), who used the extinction coefficents for Chla (16.75 l g−1 cm−1 and 82.04 l g−1 cm−1 at 645 nm and 663 nm, respectively) and Chlb (45.6 l g−1 cm−1 and 9.27 l g−1 cm−1 at 645 nm and 663 nm, respectively) given by MacKinney (1941). The soluble proteins were extracted by grinding frozen leaf subsamples to a fine powder in 50 mM N-[tri(hydroxymethyl)methyl] glycine (Tricine) buffer (pH 8.0), 2 mM EDTA, 10 mM NaCl, 5 mM MgCl2, 75 mM sucrose, 5 mM ε-aminocaproic acid, 2 mM benzamidine, 8 mM β-mercaptoethanol (+βme), and 2 mM PMSF for 5 min on ice. This was followed by centrifugation at 12 500 g at 4 °C for 30 min. Protein concentrations were measured in the decanted supernatant (Bradford, 1976), and 5 vols of cold acetone were added to an aliquot containing 200 mg of protein, which was left overnight in the freezer. The samples were then centrifuged at 12 000 g at 4 °C for 15 min. The acetone was allowed to evaporate off. The precipitates were dissolved in 65 mM TRIS-HCl (pH 6.8), 3 M sucrose, 0.6 M βme, 5% sodium dodecylsulphate (SDS, w/v), and 0.01% bromophenol blue at 96 °C for 7 min. The samples were then cooled to room temperature and aliquots of the SDS-dissociated extracts, containing 15 μg of protein, were loaded onto a 12.5% SDS–polyacrylamide gel (Mini-Protean 3 Cell, Bio Rad). This protein amount was within the range of linear response of optical density to the concentration of BSA standard (66 kDa), according to previous calibration measurements. The solubilized proteins were separated by SDS–PAGE (Laemmli, 1970) using a 0.75 mm thick gel (12.5% resolving, 4% stacking). Electrophoresis was carried out at room temperature at a constant 200 V. The gels were fixed in 500:150:75 (v/v/v) water–methanol–acetic acid mixture for 75 min, stained in EZ Blue Gel Staining (Sigma) solution for 2 h, and subsequently rinsed in water to remove excess stain. Finally, the gels were scanned with a high-resolution scanner (Scanjet G4050, Hewlett Packard, Spain) and the amount of Rubisco subunits was determined by densitometry with image analysis software (Image Quant, Molecular Dynamics, GE Healthcare, Spain). Alternatively, when electrophoresis with non-reducing (–βme) gels was performed, the frozen leaf samples were extracted in buffer without βme containing 10 mM iodoacetamide to prevent the formation of disulphide bonds, before the addition of SDS loading buffer without βme and boiling. To a separate aliquot, βme was added to a final concentration of 0.6 M, as a control of the same samples under reducing (+βme) conditions (Marín-Navarro and Moreno, 2006).
Following electrophoresis, additional gels were blotted for 75 min to PVDF membranes (Bio-Rad, Madrid, Spain) pre-wetted in methanol and equilibrated in 25 mM TRIS, 192 mM glycine, 20% methanol, and 0.1% SDS (pH 8.3) using an electrotransfer cell (Mini Trans-Blot, Bio-Rad, Madrid, Spain) at 400 mA. The blots were blocked immediately following transfer in 2% ECL Advance blocking reagent (GE Healthcare, Barcelona, Spain) in 20 mM TRIS, 137 mM NaCl (pH 7.6) with 0.1% (v/v) Tween-20 (TBS-T) for 1 h at room temperature with shaking. Blots were briefly rinsed twice in TBS-T, then probed with 1:50 000 diluted polyclonal antibody specific for the large Rubisco subunit (Rubisco quantitation kit, Agrisera, Vännäs, Sweden) for 1 h at room temperature with shaking. The antibody solution was decanted and the blot was briefly rinsed twice, and then washed once for 15 min and three times for 5 min in TBS-T at room temperature with shaking. Next, the blots were incubated in secondary antibody (anti-chicken Ig Y peroxidase conjugate, Sigma, Spain) diluted at 1:160 000 in 2% ECL Advance blocking solution for 1 h at room temperature with shaking. The blots were then washed as above and developed for 5 min with ECL Advance detection reagent according to the manufacturer's instructions. Images of the blots were obtained using a CCD imager (Fluor-S Multilmager, Bio-Rad).
Statistical analyses
One-way analyses of variance with sampling time as a factor were carried out with the GenStat 6.2 (VSN International Ltd, Hemel Hempstead, UK) statistical software. From these analyses, the standard errors of the differences (SEDs), and the least significant differences of means (three replicates) at P <0.05 probability were derived. The latter were used for the inspection of differences among values for each sampling time. The homogeneity of variance and the significance of the analysis were not modified appreciably by using arcsine-square root transformation of percentage variables (Rubisco activation and percentage in soluble protein). Therefore, the untransformed data were used.
Results
Water and Chl contents
Leaf RWC (Table 1) increased sharply from 3.4% to 87.0% during the first 12 h of rehydration, and additional water was gained until full turgor was reached at 96 h after the start of rehydration. Concomitant changes in specific leaf area, with a maximum of ∼0.17 cm2 mg−1 dry weight by 12 h and little further change, have been reported previously (Tuba et al., 1993b). Chl contents (Table 1) were low in desiccated leaves, Chlb being relatively more abundant than Chla. Chl contents rose during the rehydration period, with a faster increase after 24 h. Chla accumulated to a greater extent than Chlb, such that the Chla:Chlb ratios, which were initially <1, reached a value >2.5—within the range found in other plants—by 48 h.
Table 1.
Relative water contents (RWC), Chl contents, and Chl fluorescence parameters in leaves of Xerophyta scabrida during rehydration
| Parameter | Time of rehydration (h) |
P | SED | |||||
| 0 | 12 | 24 | 48 | 72 | 96 | |||
| RWC (%) | 3.4 a | 87 b | 92 c | 96 d | 97 e | 100 f | <0.001 | 0.04 |
| Chla (mg g−1 t. wt) | 0.010 a | 0.014 a | 0.047 a | 0.19 b | 0.29 b | 0.43 c | <0.001 | 0.054 |
| Chlb (mg g−1 t. wt) | 0.039 a | 0.049 a | 0.043 a | 0.070 a,b | 0.11b | 0.17 c | <0.001 | 0.023 |
| Chla:Chlb | 0.13 a | 0.18 a | 1.1 b | 2.7 c | 2.6 c | 2.5 c | <0.001 | 0.24 |
| Chl a+b (mg g−1 t. wt) | 0.049 a | 0.063 a | 0.090 a | 0.26 b | 0.41 b | 0.60 c | <0.001 | 0.074 |
| Fo | 0.015 a | 0.13 c | 0.090 b | 0.095 b | 0.091 b | <0.001 | 0.012 | |
| Fm | 0.020 a | 0.25 b | 0.36 c | 0.42 c | 0.45 c | <0.001 | 0.037 | |
| Fv/Fm | 0.21 a | 0.44 b | 0.75 c | 0.77 c | 0.80 c | <0.001 | 0.085 | |
| Fq'/Fm' | 0 a | 0.047 b | 0.086 c | 0.099 c | 0.004 | 0.017 | ||
| Fv'/Fm' | 0.28 a | 0.50 b | 0.53 b | 0.56 b | 0.041 | 0.072 | ||
| Fq'/Fv' | 0 a | 0.10 b | 0.16 b | 0.18 b | 0.01 | 0.034 | ||
| qL | 0 a | 0.060 a | 0.081 a | 0.088 a | 0.064 | 0.025 | ||
| ΦNPQ | 0.60 a | 0.67 a | 0.69 a | 0.67 a | 0.26 | 0.034 | ||
| ΦNO | 0.40 b | 0.28 a | 0.23 a | 0.24 a | 0.017 | 0.037 | ||
Chl fluorescence parameters in the light were measured at 1500 μmol m−2 s−1 light intensity.
P, probability in the analysis of variance; SED, standard error of the difference among means (n=3); within each row, values with the same letter are not significanty different; t. wt, turgid weight.
Chl fluorescence
The maximum quantum efficiency of PSII photochemistry (Fv/Fm, Table 1) increased from 12 h to 48 h of rehydration and changed little thereafter. This change was a consequence of a small increase in Fo and a large increase in Fm. The lack of variable fluorescence in the light-adapted state prevented the determination of the fluorescence parameters until 24 h of rehydration. In illuminated leaves (1500 μmol m−2 s−1), the PSII operating efficiency and the efficiency factor (Fq′/Fm′ and Fq′/Fv′, respectively; Table 1) underwent increases from 24 h to 72 h of rehydration, while Fv′/Fm′ rose from 24 h to 48 h with little further change, as was the case for Fv/Fm. The pattern of change with time in the fraction of open PSII centres (qL) was similar to that in Fq′/Fv′, but with lower absolute values. The quantum yield of non-photochemical quenching (ΦNPQ, Table 1) underwent little change during the rehydration period, while the quantum yield of non-radiative decay (ΦNO) decreased by 43% from 24 h to 72 h. By comparison, a rise in NPQ was observed in this interval (data not shown).
CO2 fixation
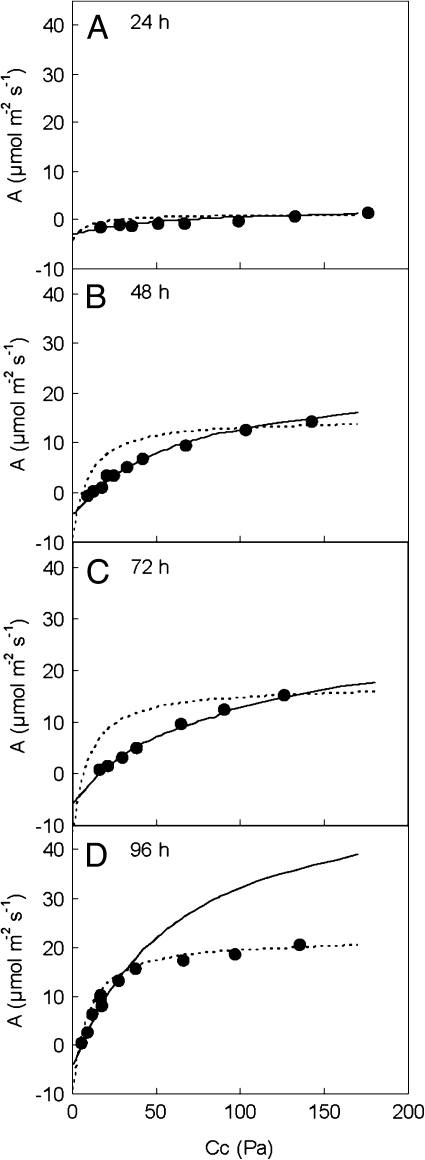
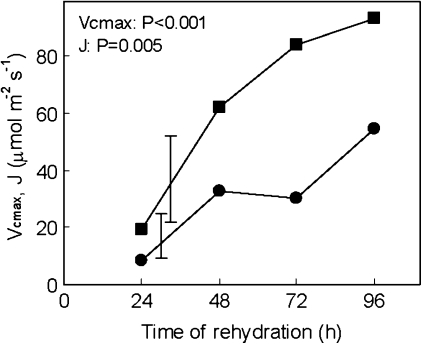
Regardless of the CO2 concentration used in measurements, there was no CO2 uptake at 24 h of rehydration (Fig. 1A) or before. Photosynthesis increased in the following 3 d rehydration period and was Rubisco limited up to very high chloroplast CO2 concentrations at 48 h and 72 h (Fig. 1B, C). The transition from Rubisco-limited to RuBP regeneration-limited photosynthesis decreased to 33 Pa CO2 partial pressure at 96 h (Fig. 1D), which is still high in comparison with other plants. Vcmax and J were calculated (Fig. 2) from the photosynthesis–CO2 response curves. Except for a drop in Vcmax at 72 h, which can be attributed to a variation between samples, both J and Vcmax increased from 24 h to 96 h, without reaching a plateau. There were relatively higher increases in J than in Vcmax.
Fig. 1.
Photosynthetic responses of Xerophyta scabrida leaves to the CO2 concentration inside the chloroplast during rehydration. Observed data (filled circles); Rubisco-limited photosynthesis (solid line); RuBP regeneration-limited photosynthesis (dotted line). Measurements were performed under 1500 μmol m−2 s−1 irradiance, at 25 °C and 1.6±0.23 kPa vapour pressure deficit. The statistical significance of the parameters derived from this figure is shown in Fig. 2.
Fig. 2.
Maximum carboxylation rate allowed by Rubisco, Vcmax (filled circles), and the rate of photosynthetic electron transport, J (filled squares), in Xerophyta scabrida leaves during rehydration. Vertical bars represent least significant differences between means (n=3).
Rubisco activity and contents
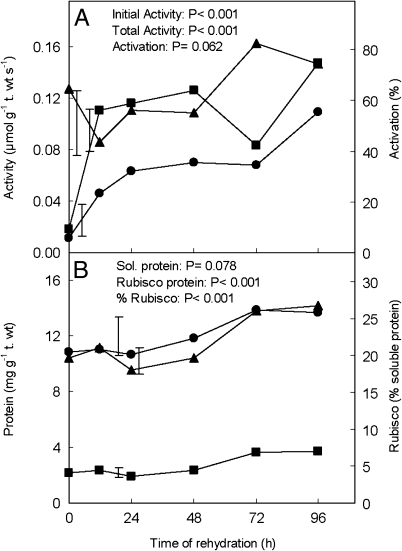
No Rubisco activity was detected in assays carried out at room temperature, but activity was recorded when the enzyme activation and assays were performed at 35 °C (Fig. 3A). Both the initial and total Rubisco activities increased during the 96 h hydration period, with a faster rise—from 12% to 75% final, total Rubisco activity—during the first 12 h. The activation of the enzyme was 44–64% in the first 48 h and increased to 74–83 % in the last 2 d.
Fig. 3.
Changes during rehydration of Xerophyta scabrida leaves in (A) initial (filled circles) and total (filled squares) Rubisco activities and Rubisco activation state (filled triangles) measured at 35 °C; and (B) Rubisco (filled squares) and total soluble proteins (filled circles) and amount of Rubisco as a percentage of soluble protein (filled triangles). Rubisco was quantified by densitometric analysis of +βme SDS–PAGE. Values are expressed on a turgid weight basis. Vertical bars represent least significant differences between means (n=3).
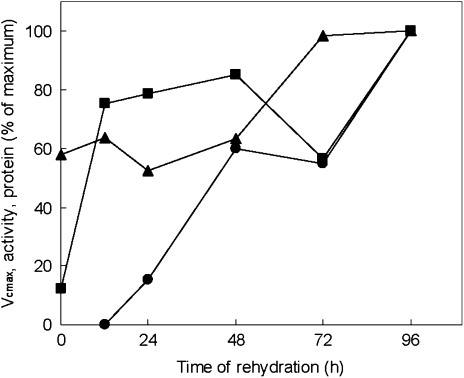
Rubisco protein amounts (Fig. 3B) were quantified by SDS–PAGE densitometric analysis of samples extracted with +βme (see Materials and methods). There was little change in the amount of Rubisco protein during the first 48 h of rehydration, although this was followed by an increase of ∼56%. Similarly, total soluble protein remained unchanged for 48 h and then increased by ∼18% (Fig. 3B). As a fraction of soluble protein, Rubisco was relatively low initially and increased (from 19% to 27%) in the last 2 d of rehydration. When Rubisco protein contents and in vitro and in vivo estimated (Vcmax) total Rubisco activities were compared (Fig. 4), it was found that the continued increase in the latter was accompanied by relatively smaller changes in total in vitro Rubisco activity after 12 h of rehydration and by an increase in Rubisco protein only after 48 h.
Fig. 4.
Effect of rehydration of Xerophyta scabrida leaves on Vcmax (filled circles), in vitro total Rubisco activity (filled squares), and Rubisco protein (filled triangles), as a percentage of values at 96 h.
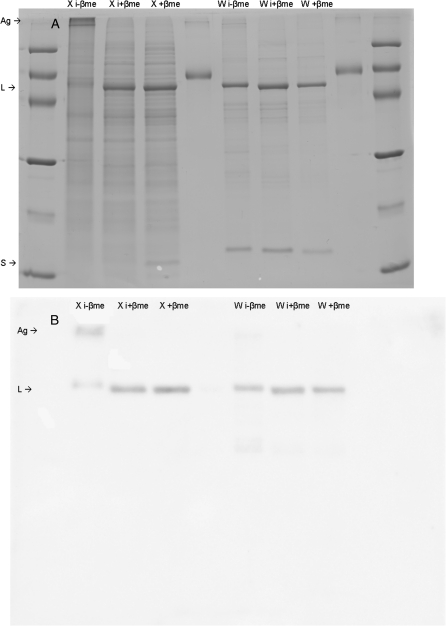
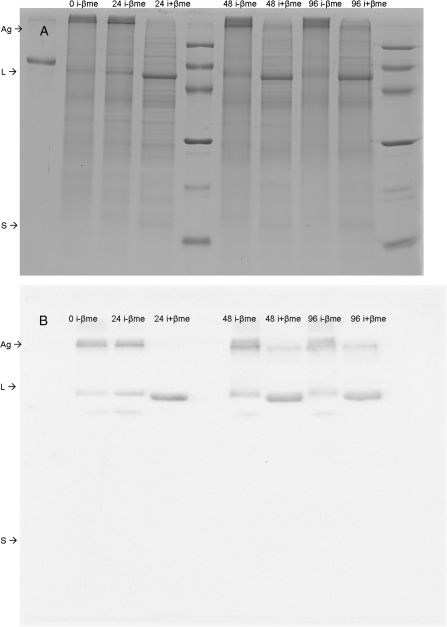
To examine further the disparity between Vcmax and Rubisco contents, the electrophoresis of samples extracted either with +βme or with iodoacetamide and –βme to block the sulphydryl groups was compared. Whereas with the reducing agent the two Rubisco subunits migrated according to their molecular weights (Fig. 5), with blocked sulphydryl groups the Rubisco remained at the top of the gel. In separate aliquots of the same samples to which βme had been added following iodoacetamide treatment, the large Rubisco subunit showed normal migration in the gels, but the small Rubisco subunit was not observed. In comparison, wheat samples extracted with βme or with iodoacetamide with either +βme or –βme showed few electrophoretic differences, the two Rubisco subunits undergoing normal migration. The examination of the iodoacetamide –βme and +βme gel electrophoresis (Fig. 6) revealed that in both desiccated and rehydrating X. scabrida leaves Rubisco formed high molecular weight aggregates. It was therefore investigated whether the reversible aggregation of Rubisco might account for the differences between the gas exchange measurements and Rubisco contents. Leaves harvested at each sampling time were analysed immediately after illumination with bright light in moistened air, rather than after an equilibration period in water after the photosynthesis measurements (see Materials and methods). In these leaves, Rubisco remained in an aggregated state (data not shown).
Fig. 5.
Soluble proteins in desiccated Xerophyta scabrida (X) and fresh wheat (W) leaves. (A) SDS–PAGE and (B) immunoblotting of samples extracted in buffer containing 10 mM iodoacetamide without β-mercaptoethanol (i-βme), containing iodoacetamide to which βme had been added prior to electrophoresis (i+βme), or with βme without iodoacetamide (+βme). Molecular markers were loaded onto lanes 1 and 10 and BSA standard onto lanes 5 and 9. Rubisco aggregates (Ag) and large (L) and small (S) subunits are indicated on the left.
Fig. 6.
Soluble proteins in Xerophyta scabrida leaves after different rehydration periods. (A) SDS–PAGE and (B) immunoblotting of samples extracted in buffer containing 10 mM iodoacetamide without β-mercaptoethanol (i-βme), or containing iodoacetamide to which βme had been added prior to electrophoresis (i+βme). Leaves desiccated (0) or rehydrated for 24 h (24), 48 h (48), or 96 h (96). Molecular markers were loaded onto lanes 5 and 10, and BSA standard onto lane 1. Rubisco aggregates (Ag) and large (L) and small (S) subunits are indicated on the left.
Discussion
The rehydration of leaves and the recovery of Chl contents in this experiment were in general agreement with earlier reports on poikilochlorophyllous resurrection plants (Tuba et al., 1994; Proctor and Tuba, 2002; Degl'Innocenti et al., 2008). It may be remarked that Chlb was relatively more abundant than Chla in desiccated leaves, but by 48 h the Chla:Chlb ratio was >2.0. This suggests a rise during rehydration in the PSII reaction centres with respect to the antenna complexes (Habash et al., 1995; Bailey et al., 2001), which is consistent with the reported up-regulation of PSII genes (Ingle et al., 2007, 2008).
The PSII operating efficiency (Fq′/Fm′), in contrast to Fv/Fm and Fv′/Fm′, was still relatively low at 48 h after the start of rehydration and continued to increase up to the following day, as did the PSII efficiency factor. Fq′/Fv′ is generally much more affected by the ability to utilize the products of the electron transport chain than by changes in NPQ (Baker et al., 2007). This points to the capacity for carbon assimilation as the factor that limits the efficiency at which the light absorbed by PSII is used for photochemistry in rehydrating X. scabrida leaves. A similar conclusion was reached for the HDT H. rodopensis during dehydration (Georgieva et al., 2005). Fq′/Fv′ is non-linearly related to the proportion of PSII centres that are in the open state (with the primary quinone electron acceptor of PSII, QA, oxidized), as estimated by qL (Baker et al., 2007). Changes during rehydration in this parameter and Fq′/Fv′ were parallel and showed an increasing fraction of open PSII centres as the carboxylation capacity increased. In contrast to previous reports on the engagement of non-photochemical energy dissipation upon the remoistening of DT leaves (Csintalan et al., 1999; Augusti et al., 2001; Degl'Innocenti et al., 2008), the lack of significant changes in ΦNPQ suggests that the down-regulation of PSII was not a major cause of the changes observed during rehydration in the maximum efficiency of PSII in the light. The significant decline in ΦNO probably reflects the reconstitution of functional PSII antennae and reaction centres.
The capacity for carbon assimilation was also recovered during rehydration, although the assimilation rates could differ from those of intact plant leaves due to signalling and metabolic interactions with other organs. In agreement with the results concerning Chl fluorescence, the responses of carbon assimilation to the CO2 concentration in the chloroplast (Figs 1, 2) indicated that the recovery of photosynthetic capacity during rehydration was relatively more limited by carboxylation than by the rate of electron transport. Rubisco activity may therefore be of paramount importance for the photosynthetic competence of rehydrated desiccation-tolerant plants. The present results show that a significant fraction of the Rubisco protein found in rehydrated leaves is present in desiccated leaves (Fig. 3), and that new synthesis occurs later in the process of rehydration. Notably, in both desiccated and rehydrated X. scabrida leaves—in contrast to wheat—Rubisco was in an aggregated state (Fig. 5), as in L. minor fronds under osmotic stress (Ferreira and Shaw, 1989). Our gas exchange measurements and Rubisco activity assays revealed that free or membrane-bound Rubisco aggregates in X. scabrida were inactive in desiccated leaves and in the early rehydration stages. Treatments such as high light intensity in gas exchange analysis or mild warming under reducing conditions in activity assays rendered Rubisco progressively more active. This suggests that the integrity of Rubisco was preserved in the aggregates, but that a modification required for the enzyme to become functional was facilitated by rehydration. In dormant Retama raetam tissues, Rubisco and other proteins also appear to be present as high molecular weight complexes (Pnueli et al., 2002). These complexes precipitated during extraction with reducing buffers, a result that was observed for the small Rubisco subunit only when βme was added to extracts containing iodoacetamide. Pnueli et al. (2002) suggested that the dilution of reducing equivalents upon rehydration releases proteins from the aggregates into their soluble, active form. However, some of the DTT concentrations used by Pnueli et al. (2002) in the protein extraction buffer have been shown to cause Rubisco aggregation and precipitation (Cho et al., 2008). Moreover, the present results suggested that the increase in Rubisco activity during rehydration was not associated with protein release from the aggregates. The lower oxidation states of thiol groups (disulphide and sulphenic acid) may easily be reverted again to the sulphydryl state by disulphide exchange with free thiols, by DTT (in vitro) or by thioredoxins and glutaredoxins (Marcus et al., 2003; Moreno et al., 2008). It is possible that oxidative conditions during desiccation could induce the formation of disulphides in the Rubisco molecule, and that the recovery of photochemical activity could lead to an increasingly reduced stroma, favouring the reductive activation of Rubisco. While upon desiccation of X. scabrida, and indeed of all poikilochlorophyllous plant species, Chl and the photosynthetic apparatus are lost, it is concluded that Rubisco is preserved in large amounts in a close to functional state. Rubisco aggregation may be a part of the poikilochlorophylly strategy.
Acknowledgments
This work has been funded by the Spanish Ministry of Science and Innovation and the Hungarian Science and Technology Office (Integrated Action HH2006-0019, ESP-41/2006), and by the Spanish National Research and Development Programme-European Regional Development Fund ERDF (Project AGL2006-13541-C02-02/AGR). DG was the recipient of a Junta de Castilla y León fellowship. This paper is dedicated to the memory of Zoltán Tuba, who passed away while this research was in progress.
Glossary
Abbreviations
- Chl
chlorophyll
- DT
desiccation tolerant
- HDT
homoiochlorophyllous DT
- PDT
poikilochlorophyllous DT
- RuBP
ribulose-1,5-bisphosphate
- RWC
relative water content
References
- Agarwal R, Ortlebb S, Sainis JK, Melzer M. Immunoelectron microscopy for locating Calvin cycle enzymes in the thylakoids of Synechocystis 6803. Molecular Plant. 2009;2:32–42. doi: 10.1093/mp/ssn075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson LE, Goldhaber-Gordo IM, Li D, Tang XY, Xiang M, Prakash N. Enzyme–enzyme interaction in the chloroplast, glyceradehyde-3-phosphate dehydrogenase, triose phosphate isomerase and aldolase. Planta. 1995;196:245–255. doi: 10.1007/BF00201381. [DOI] [PubMed] [Google Scholar]
- Arnon DI. Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiology. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti A, Scartazza A, Navari-Izzo F, Sgherri CLM, Stevanovic B, Brugnoli E. Photosystem II photochemical efficiency, zeaxanthin and antioxidant contents in the poikilohydric Ramonda serbica during dehydration and rehydration. Photosynthesis Research. 2001;67:79–88. doi: 10.1023/A:1010692632408. [DOI] [PubMed] [Google Scholar]
- Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta. 2001;213:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- Baker NR, Harbinson J, Kramer DM. Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant, Cell and Environment. 2007;30:1107–1125. doi: 10.1111/j.1365-3040.2007.01680.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cho JH, Hwang H, Cho MH, Kwon YK, Jeon JS, Bhoo SH, Hahn TR. The effect of DTT in protein preparations for proteomic analysis: removal of a highly abundant plant enzyme, ribulose bisphosphate carboxylase/oxygenase. Journal of Plant Biology. 2008;51:297–301. [Google Scholar]
- Csintalan Zs, Proctor MCF, Tuba Z. Chlorophyll fluorescence during drying and rehydration in the mosses Rhytidiadelphus loreus (Hedw.) Warnst., Anomodon viticulosus (Hedw.) Hook. & Tayl. and Grimmia pulvinata (Hedw.) Sm. Annals of Botany. 1999;84:235–244. [Google Scholar]
- Daniel V, Gaff DF. Sulfhydryl and disulphide levels in protein fractions from hydrated and dry leaves of resurrection plants. Annals of Botany. 1980;45:163–171. [Google Scholar]
- Degl'Innocenti E, Guidi L, Stevanovic B, Navari F. CO2 fixation and chlorophyll a fluorescence in leaves of Ramonda serbica during a dehydration–rehydration cycle. Journal of Plant Physiology. 2008;165:723–733. doi: 10.1016/j.jplph.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Ferreira RB, Shaw NM. Effect of osmotic stress on protein turnover in Lemna minor fronds. Planta. 1989;179:456–465. doi: 10.1007/BF00397585. [DOI] [PubMed] [Google Scholar]
- Frank W, Phillips J, Salamini F, Bartels D. Two dehydration-inducible transcripts from the resurrection plant Craterostigma plantagineum encode interacting homeodomain leucine zipper proteins. The Plant Journal. 1998;15:413–421. doi: 10.1046/j.1365-313x.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- Georgieva K, Maslenkova L, Peeva V, Markovska Y, Stefanov D, Tuba Z. Comparative study on the changes in photosynthetic activity of the homoiochlorophyllous desiccation-tolerant Haberlea rhodopensis and desiccation-sensitive spinach leaves during desiccation and rehydration. Photosynthesis Research. 2005;85:191–203. doi: 10.1007/s11120-005-2440-0. [DOI] [PubMed] [Google Scholar]
- Georgieva K, Szigeti Z, Sarvari E, Gaspar L, Maslenkova L, Peeva V, Peli E, Tuba Z. Photosynthetic activity of homoiochlorophyllous desiccation tolerant plant Haberlea rhodopensis during dehydration and rehydration. Planta. 2007;225:955–964. doi: 10.1007/s00425-006-0396-8. [DOI] [PubMed] [Google Scholar]
- Gontero B, Cardenas M, Ricard J. A functional five enzyme complex of chloroplasts involved in the Calvin cycle. European Journal of Biochemistry. 1988;173:437–443. doi: 10.1111/j.1432-1033.1988.tb14018.x. [DOI] [PubMed] [Google Scholar]
- Gontero B, Mulliert G, Rault M, Giudico-Orticoni MT, Ricard J. Structural and functional properties of a multi-enzyme complex from spinach chloroplasts: modulation of the kinetic properties of enzymes in the aggregated state. European Journal of Biochemistry. 1993;217:1075–1082. doi: 10.1111/j.1432-1033.1993.tb18339.x. [DOI] [PubMed] [Google Scholar]
- Gutiérrez D, Gutiérrez E, Pérez P, Morcuende R, Verdejo AL, Martinez-Carrasco R. Acclimation to future atmospheric CO2 increases photochemical efficiency and mitigates photochemistry inhibition by warm temperatures in wheat under field chambers. Physiologia Plantarum. 2009;137:86–100. doi: 10.1111/j.1399-3054.2009.01256.x. [DOI] [PubMed] [Google Scholar]
- Habash DZ, Matthew JP, Parry MAJ, Keys AJ, Lawlor DW. Increased capacity for photosynthesis in wheat grown at elevated CO2: the relationship between electron transport and carbon metabolism. Planta. 1995;197:482–489. [Google Scholar]
- Harten JB, Eickmeier WG. Enzyme dynamics of the resurrection plant Selaginella lepidophylla (Hook. & Grev.) Spring during rehydration. Plant Physiology. 1986;82:61–64. doi: 10.1104/pp.82.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermoso R, Fonollá J, de Felipe MR, Vivo MA, Chueca A, Lázaro J, Lopez Gorgé J. Double immunogold localization of thioredoxin f and photosynthetic fructose-1,6-bisphosphatase in spinach leaves. Plant Physiology and Biochemistry. 1992;30:39–46. [Google Scholar]
- Ingle RA, Collett H, Cooper K, Takahashi Y, Farrant JM, Illing N. Chloroplast biogenesis during rehydration of the resurrection plant Xerophyta humilis: parallels to the etioplast–chloroplast transition. Plant, Cell and Environment. 2008;31:1813–1824. doi: 10.1111/j.1365-3040.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- Ingle RA, Schmidt UG, Farrant JM, Thomson JA, Mundree SG. Proteomic analysis of leaf proteins during dehydration of the resurrection plant Xerophyta viscosa. Plant, Cell and Environment. 2007;30:435–446. doi: 10.1111/j.1365-3040.2006.01631.x. [DOI] [PubMed] [Google Scholar]
- Ingram J, Bartels D. The molecular basis of dehydration tolerance in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47:377–403. doi: 10.1146/annurev.arplant.47.1.377. [DOI] [PubMed] [Google Scholar]
- Klughammer C, Schreiber U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the saturation pulse method. PAM Application Notes. 2008;1:27–35. [Google Scholar]
- Kramer DM, Johnson G, Kiirats O, Edwards GE. New fluorescence parameters for determination of QA redox state and excitation energy fluxes. Photosynthesis Research. 2004;79:209–218. doi: 10.1023/B:PRES.0000015391.99477.0d. [DOI] [PubMed] [Google Scholar]
- Kranner I, Beckett RP, Wornik S, Zorn M, Pfeifhofer HW. Revival of a resurrection plant correlates with its antioxidant status. The Plant Journal. 2002;31:13–24. doi: 10.1046/j.1365-313x.2002.01329.x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilley R McC, Walker DA. An improved spectrophotometric assay for ribulose bisphosphate carboxylase. Biochimica et Biophysica Acta. 1974;358:226–229. doi: 10.1016/0005-2744(74)90274-5. [DOI] [PubMed] [Google Scholar]
- MacKinney G. Absorption of light by chlorophyll solutions. Journal of Biological Chemistry. 1941;140:315–322. [Google Scholar]
- Marcus Y, Altman-Gueta H, Finkler A, Gurevitz M. Dual role of cysteine 172 in redox regulation of ribulose 1,5-bisphosphate carboxylase/oxygenase activity and degradation. Journal of Bacteriology. 2003;185:1509–1517. doi: 10.1128/JB.185.5.1509-1517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Navarro J, Moreno J. Cysteines 449 and 459 modulate the reduction–oxidation conformational changes of ribulose 1,5-bisphosphate carboxylase/oxygenase and the translocation of the enzyme to membranes during stress. Plant, Cell and Environment. 2006;29:898–908. doi: 10.1111/j.1365-3040.2005.01469.x. [DOI] [PubMed] [Google Scholar]
- Martinelli T, Whittaker A, Masclaux-Daubresse C, Farrant JM, Brilli F, Loreto F, Vazzana C. Evidence for the presence of photorespiration in desiccation-sensitive leaves of the C4‘resurrection’ plant Sporobolus stapfianus during dehydration stress. Journal of Experimental Botany. 2007;58:3929–3939. doi: 10.1093/jxb/erm247. [DOI] [PubMed] [Google Scholar]
- Moreno J, García-Murria MJ, Marín-Navarro J. Redox modulation of Rubisco conformation and activity through its cysteine residues. Journal of Experimental Botany. 2008;59:1605–1614. doi: 10.1093/jxb/erm310. [DOI] [PubMed] [Google Scholar]
- Mowla SB, Thomson JA, Farrant JM, Mundree SG. A novel stress-inducible antioxidant enzyme identified from the resurrection plant Xerophyta viscosa Baker. Planta. 2002;215:716–726. doi: 10.1007/s00425-002-0819-0. [DOI] [PubMed] [Google Scholar]
- Peeva V, Cornic G. Leaf photosynthesis of Haberlea rhodopensis before and during drought. Environmental and Experimental Botany. 2009;65:310–318. [Google Scholar]
- Persson O, Johansson G. Studies of protein–protein interaction using countercurrent distribution in aqueous two phase systems. Biochemical Journal. 1989;259:863–870. doi: 10.1042/bj2590863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pnueli L, Hallak-Herr E, Rozenberg M, Cohen M, Goloubinoff P, Kaplan A, Mittler R. Molecular and biochemical mechanisms associated with dormancy and drought tolerance in the desert legume Retama raetam. The Plant Journal. 2002;31:319–330. doi: 10.1046/j.1365-313x.2002.01364.x. [DOI] [PubMed] [Google Scholar]
- Proctor MCF, Ligrone R, Duckett JG. Desiccation tolerance in the moss Polytrichum formosum: physiological and fine-structural changes during desiccation and recovery. Annals of Botany. 2007;99:75–93. doi: 10.1093/aob/mcl246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor MCF, Tuba Z. Poikilohydry and homoihydry: antithesis or spectrum of possibilities? New Phytologist. 2002;156:327–349. doi: 10.1046/j.1469-8137.2002.00526.x. [DOI] [PubMed] [Google Scholar]
- Ramanjulu S, Bartels D. Drought- and desiccation-induced modulation of gene expression in plants. Plant, Cell and Environment. 2002;25:141–151. doi: 10.1046/j.0016-8025.2001.00764.x. [DOI] [PubMed] [Google Scholar]
- Sainis JK, Harris GC. The association of d-ribulose-1,5-bisphosphate carboxylase with phosphoriboisomerase and phosphoribulokinase. Biochemical and Biophysical Research Communications. 1986;139:947–954. doi: 10.1016/s0006-291x(86)80269-8. [DOI] [PubMed] [Google Scholar]
- Sainis JK, Merriam K, Harris GC. The association of ribulose-1,5-bisphosphate carboxylase/oxygenase with phosphoribulokinase. Plant Physiology. 1989;89:368–374. doi: 10.1104/pp.89.1.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell and Environment. 2007;30:1035–1040. doi: 10.1111/j.1365-3040.2007.01710.x. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Savitch LV, Butz ND. Photometric method for routine determination of kcat and carbamylation of rubisco. Photosynthesis Research. 1991;28:41–48. doi: 10.1007/BF00027175. [DOI] [PubMed] [Google Scholar]
- Sherwin HW, Farrant JM. Differences in rehydration of three desiccation-tolerant angiosperm species. Annals of Botany. 1996;78:703–710. [Google Scholar]
- Toldi O, Tuba Z, Scott P. Vegetative desiccation tolerance: is it a goldmine for bioengineering crops? Plant Science. 2009;176:187–199. [Google Scholar]
- Tuba Z, Zs Csintalan, Szente K, Nagy Z, Grace J. Carbon gains by desiccation tolerant plants at elevated CO2. Functional Ecology. 1998B;12:39–44. [Google Scholar]
- Tuba Z, Hartmut K, Maroti I, Zs Csintalan, Pócs T. Regreening of dessicated leaves of the poikilochlorophyllous Xerophyta scabrida upon rehydration. Journal of Plant Physiology. 1993b;142:103–108. [Google Scholar]
- Tuba Z, Lichtenthaler HK, Csintalan Z, Nagy Z, Szente K. Reconstitution of chlorophylls and photosynthetic CO2 assimilation upon rehydration of the desiccated poikilochlorophyllous plant Xerophyta scabrida (Pax) Th. Dur. et Schinz. Planta. 1994;192:414–420. [Google Scholar]
- Tuba Z, Lichtenthaler HK, Maroti I, Csintalan Zs. Resynthesis of thylakoids and functional chloroplasts in the desiccated leaves of the poikilochlorophyllous plant Xerophyta scabrida upon rehydration. Journal of Plant Physiology. 1993a;142:742–748. [Google Scholar]
- Tuba Z, Proctor MCF, Csintalan Zs. Ecophysiological responses of homoichlorophyllous and poikilochlorophyllous desiccation tolerant plants: a comparison and an ecological perspective. Plant Growth Regulation. 1998a;24:211–217. [Google Scholar]
- Tuba Z, Smirnoff N, Zs Csintalan, Nagy Z, Szente K. Respiration during slow desiccation of the poikilochlorophyllous desiccation tolerant plant Xerophyta scabrida at present-day CO2 concentrations. Plant Physiology and Biochemistry. 1997;35:381–386. [Google Scholar]
- Vicré M, Farrant JM, Driouich A. Insights into the cellular mechanisms of desiccation tolerance among angiosperm resurrection plant species. Plant, Cell and Environment. 2004;27:1329–1340. [Google Scholar]
- Ward DA, Keys AJ. A comparison between the coupled spectrophotometric and uncoupled radiometric assay for RuBP carboxylase. Photosynthesis Research. 1989;22:167–171. doi: 10.1007/BF00035447. [DOI] [PubMed] [Google Scholar]
- Whittaker A, Bochicchio A, Vazzana C, Lindsey G, Farrant JM. Changes in leaf hexokinase activity and metabolite levels in response to drying in the desiccation-tolerant species Sporobolus stapfianus and Xerophyta viscosa. Journal of Experimental Botany. 2001;52:961–969. doi: 10.1093/jexbot/52.358.961. [DOI] [PubMed] [Google Scholar]