Abstract
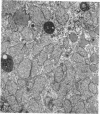
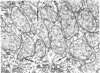
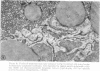
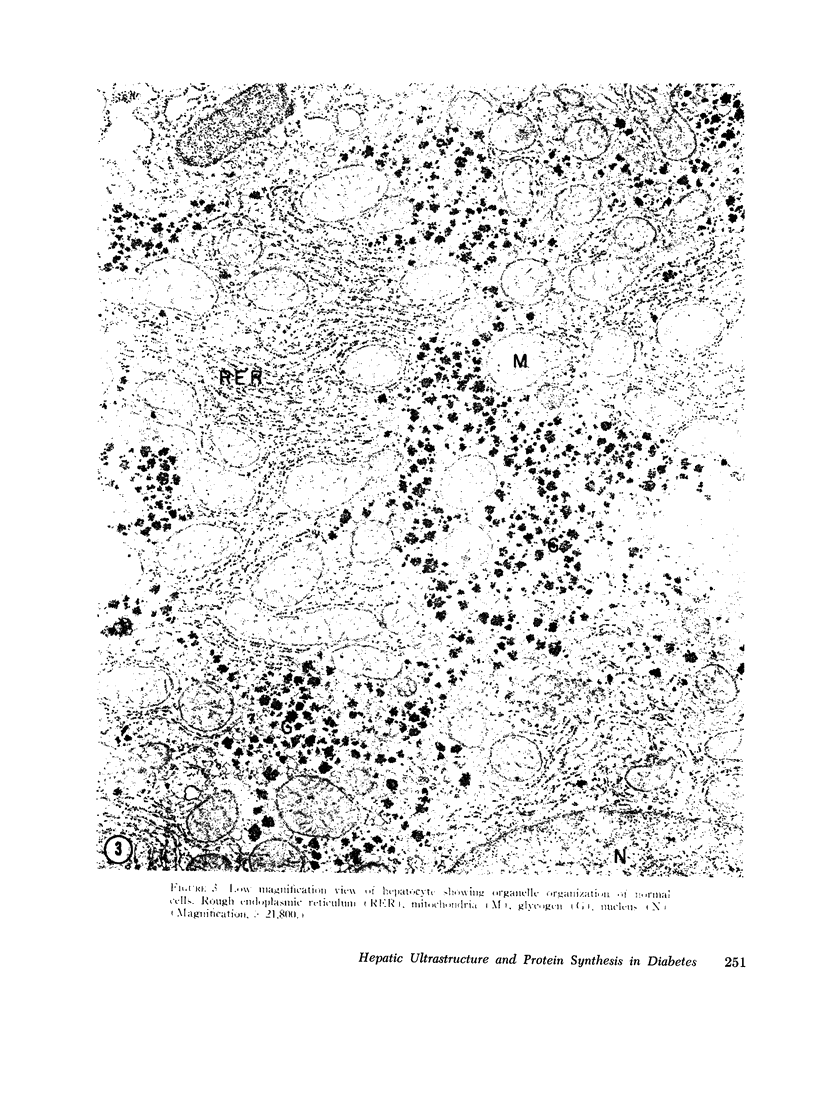
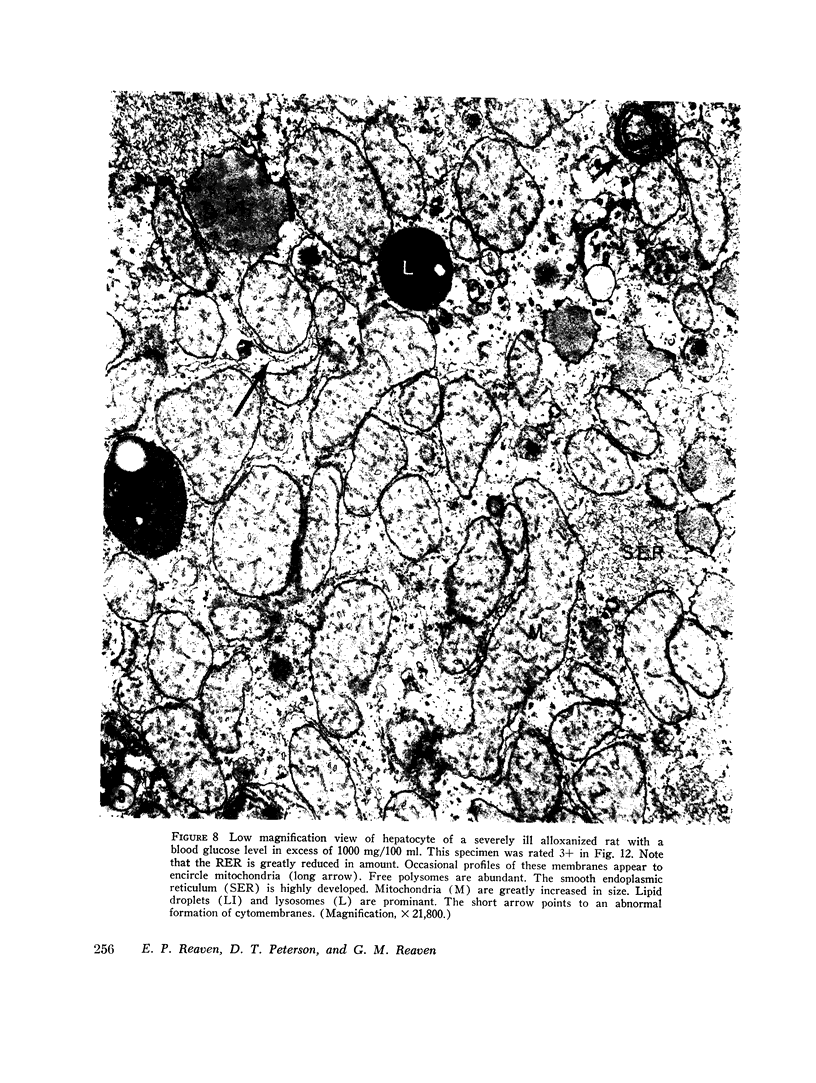
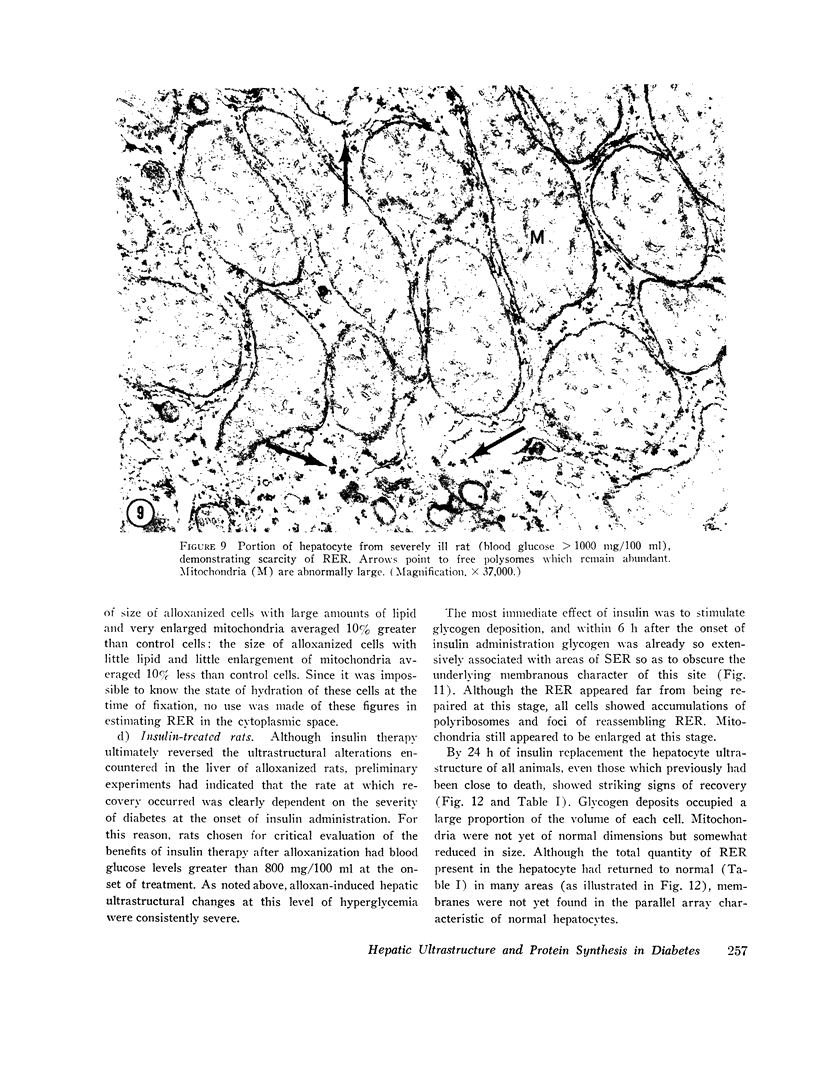
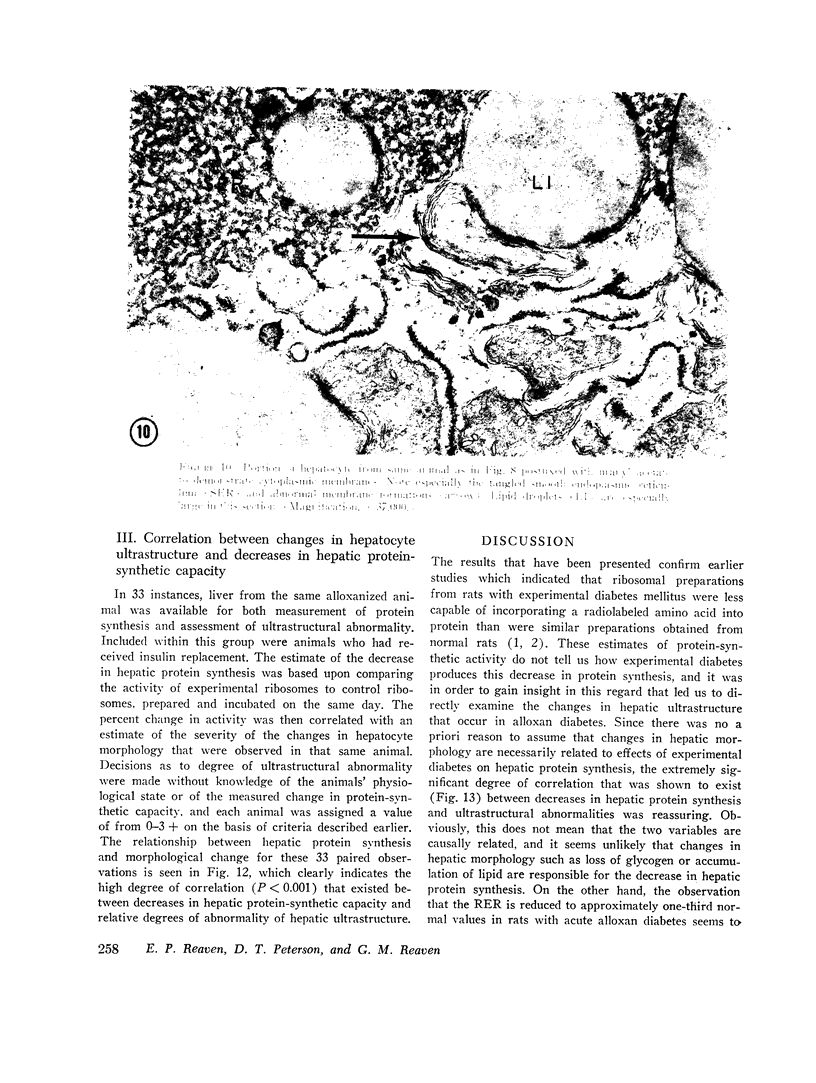
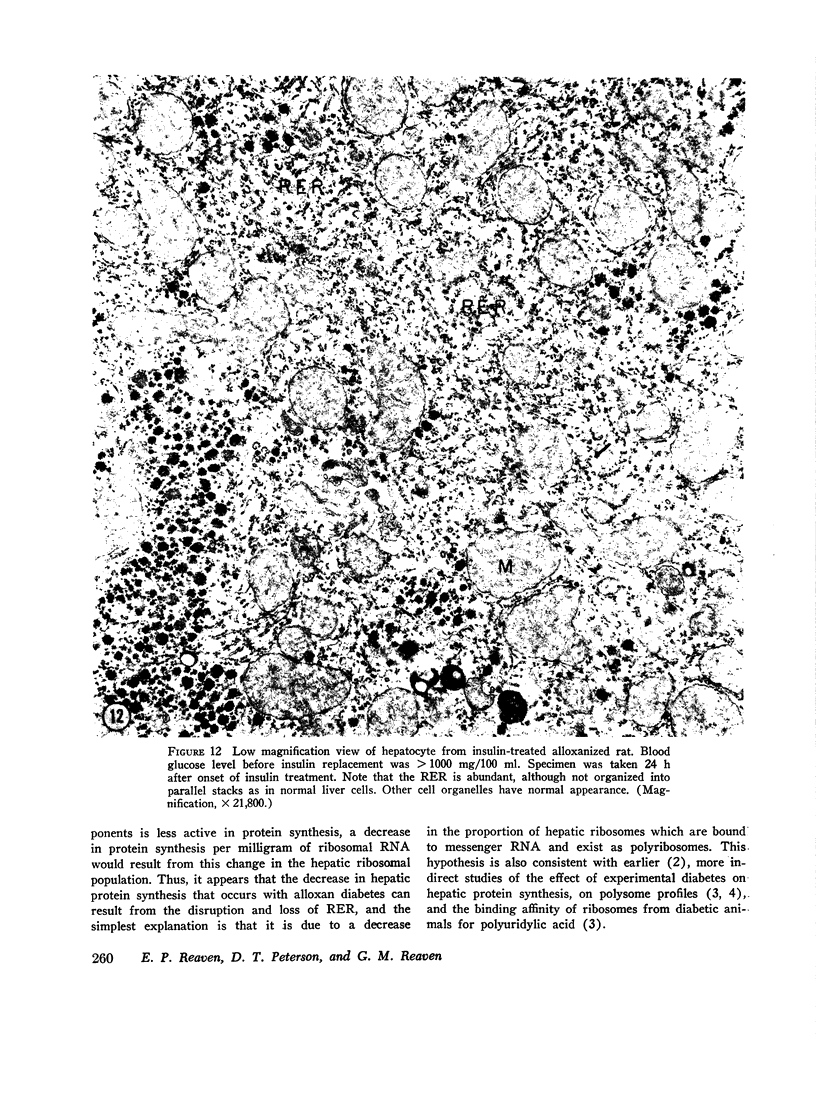
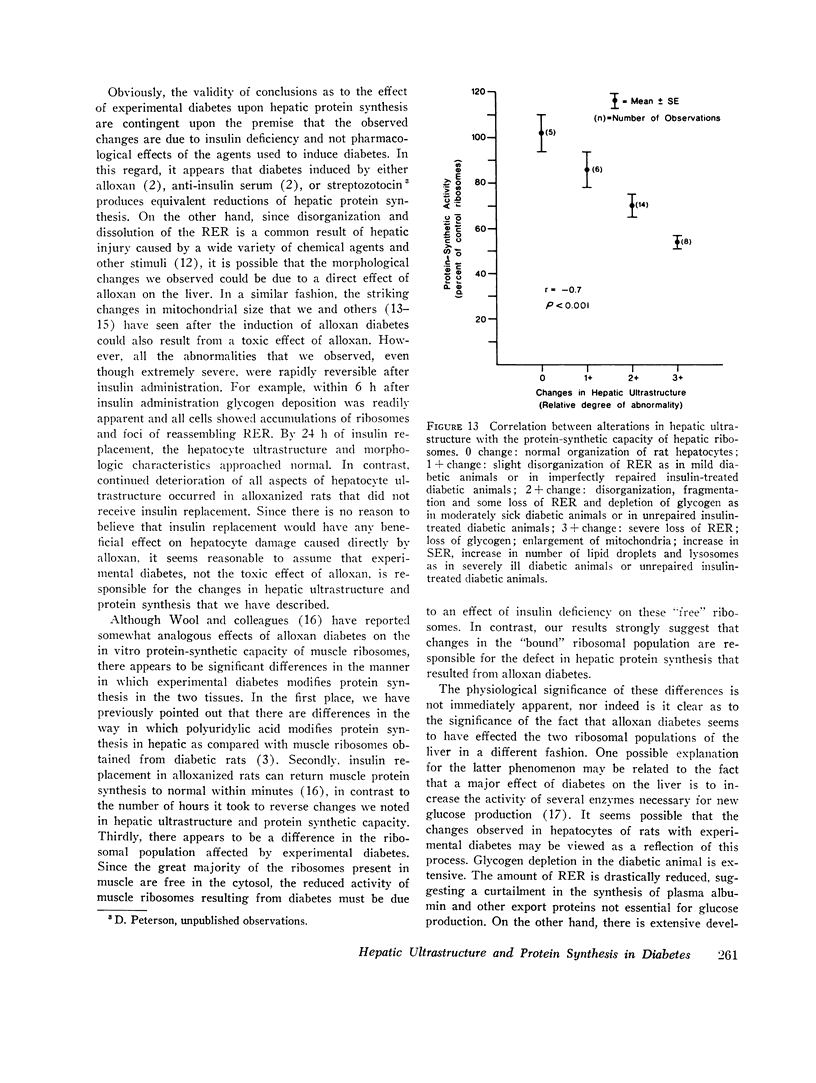
The following study was conducted in order to define the specific alterations in hepatic ultrastructure responsible for the decrease in hepatic protein synthesis associated with experimental diabetes. Rats received intravenous alloxan (70 mg/kg) and 48 h later were either sacrificed or given insulin for 1, 2, 4, 6, or 24 h. Specimens for electron microscopic evaluation and morphometric analysis were taken from the same livers used to isolate ribosomes for measurement of in vitro protein synthesis. Our results show that hepatocytes from animals with untreated alloxan diabetes show varying degrees of disorganization and loss of rough endoplasmic reticulum (RER) which is directly related to the severity of the alloxan diabetes. A significant correlation existed between the severity of ultrastructural changes as judged by the loss of both membrane and polysome components of the RER and degree of inhibition of protein synthesis (P < 0.001). Abnormalities of hepatic ultrastructure and protein synthesis were reversed within 24 h of insulin administration. The data are consistent with the view that it is the relative decrease in hepatic polysomes that results from the loss of RER in alloxan diabetes that is responsible for the decrease in hepatic protein synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruni C., Porter K. R. The Fine Structure of the Parenchymal Cell of the Normal Rat Liver: I. General Observations. Am J Pathol. 1965 May;46(5):691–755. [PMC free article] [PubMed] [Google Scholar]
- HALL J. C., SORDAHL L. A., STEFKO P. L. The effect of insulin on oxidative phosphorylation in normal and diabetic mitochondria. J Biol Chem. 1960 May;235:1536–1539. [PubMed] [Google Scholar]
- Harano Y., DePalma R. G., Miller M. Fatty acid oxidation, citric acid cycle activity, and morphology of mitochondria in diabetic rat liver. Proc Soc Exp Biol Med. 1969 Jul;131(3):913–917. doi: 10.3181/00379727-131-34008. [DOI] [PubMed] [Google Scholar]
- KORNER A. Alloxan diabetes and in vitro protein biosynthesis in rat liver microsomes and mitochondria. J Endocrinol. 1960 May;20:256–265. doi: 10.1677/joe.0.0200256. [DOI] [PubMed] [Google Scholar]
- Loud A. V. A quantitative stereological description of the ultrastructure of normal rat liver parenchymal cells. J Cell Biol. 1968 Apr;37(1):27–46. doi: 10.1083/jcb.37.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzaferri E. L., Skillman T. G., Lanese R. R., Keller M. P. Use of test strips with colour meter to measure blood-glucose. Lancet. 1970 Feb 14;1(7642):331–333. doi: 10.1016/s0140-6736(70)90706-3. [DOI] [PubMed] [Google Scholar]
- Satoh T. Electron microscopic studies on the liver and exocrine pancreas of experimental diabetic rats. Nagoya Med J. 1966 Jul;12(2):71–110. [PubMed] [Google Scholar]
- Stenger R. J. Organelle pathology of the liver. The endoplasmic reticulum. Gastroenterology. 1970 Apr;58(4):554–574. [PubMed] [Google Scholar]
- Stetten M. R., Ghosh S. B. Different properties of glucose-6-phosphatase and related enzymes in rough and smooth endoplasmic reticular membranes. Biochim Biophys Acta. 1971 Mar 9;233(1):163–175. doi: 10.1016/0005-2736(71)90369-5. [DOI] [PubMed] [Google Scholar]
- Tragl K. H., Reaven G. M. Effect of experimental diabetes mellitus on protein synthesis by liver ribosomes. Diabetes. 1971 Jan;20(1):27–32. doi: 10.2337/diab.20.1.27. [DOI] [PubMed] [Google Scholar]
- Tragl K. H., Reaven G. M. Effect of insulin deficiency on hepatic ribosomal aggregation. Diabetes. 1972 Feb;21(2):84–88. doi: 10.2337/diab.21.2.84. [DOI] [PubMed] [Google Scholar]
- Weber G., Lea M. A., Convery H. J., Stamm N. B. Regulation of gluconeogenesis and glycolysis: studies of mechanisms controlling enzyme activity. Adv Enzyme Regul. 1967;5:257–300. doi: 10.1016/0065-2571(67)90020-9. [DOI] [PubMed] [Google Scholar]
- Wittman J. S., 3rd, Lee K. L., Miller O. N. Dietary and hormonal influences on rat liver polysome profiles; fat, glucose and insulin. Biochim Biophys Acta. 1969 Feb 18;174(2):536–543. doi: 10.1016/0005-2787(69)90282-2. [DOI] [PubMed] [Google Scholar]
- Wool I. G., Stirewalt W. S., Kurihara K., Low R. B., Bailey P., Oyer D. Mode of action of insulin in the regulation of protein biosynthesis in muscle. Recent Prog Horm Res. 1968;24:139–213. doi: 10.1016/b978-1-4831-9827-9.50010-1. [DOI] [PubMed] [Google Scholar]