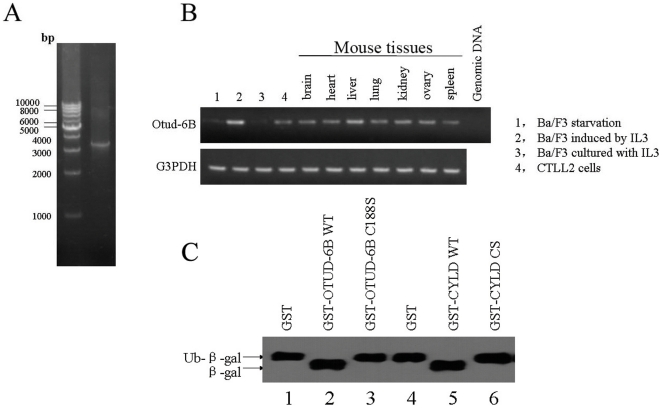
Figure 1. OTUD-6B is a functional deubiquitinating enzyme.
A. Molecular cloning of the cDNA of human OTUD-6B was amplified from total RNA of Raji cells and subjected to 1% agarose gel analysis. B. The expression of mouse Otud-6b mRNA in Ba/F3 cells [5], CTLL-2 cells and various tissues (brain, heart, liver, lung, kidney, ovary, and spleen) was analyzed by RT-PCR using Otud-6b-specific primers. G3PDH was used as control. Ba/F3 cells were kindly supplied by prof. Xin yuan Liu (Shanghai Institute of Biochemistry and Cell Biology, SIBS, CAS). CTLL2 cells were provided by the Cell Bank, Shanghai Institute of Biochemistry and Cell Biology, SIBS, CAS. C. Ub-Met-β-gal fusion protein was prepared from MC1061 cells. The supernatant was incubated with purified GST (lane 1), OTUD-6B WT (lane 2), OTUD-6B C188S (lane 3), GST (lane 4), GST-CYLD WT (lane 5), and GST-CYLD CS (lane 6) fusion protein at 4°C with rotation for 4 hours. Both OTUD-6B WT and GST-CYLD WT could cleave ubiquitin from the Ub-Met-β-gal fusion protein.