Figure 2.
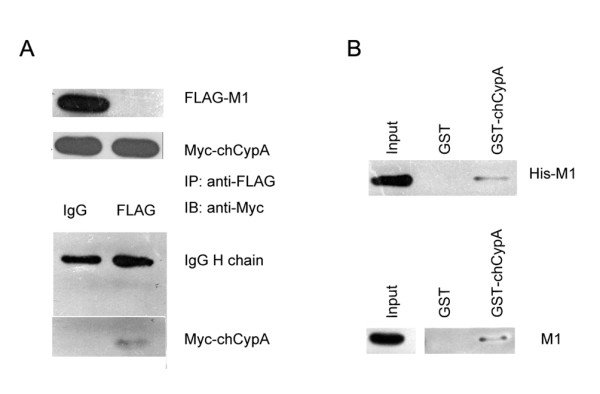
ChCypA interacted with M1 protein of influenza virus in vitro and in vivo. A. Co-immunoprecipitation of M1 and chCypA. Input shows 1/10 of the total proteins included in each binding reaction. Lane 1, pcDNA3-FLAG-M1 and pCMV-Myc-chCypA plasmids were simultaneously transfected into 293T cells. Lane 2, pCMV-Myc-chCypA plasmid was transfected in 293T cells. 48 h after transfection, the cells were lysed in Hepes buffer prepared for co-immunoprecipitation. Co-immunoprecipitation was performed using anti-FLAG monoclonal antibody, and the proteins immunoprecipitated (IP) were detected with an anti c-Myc monoclonal antibody. B. GST pull-down assay was used to detect the interaction of influenza A virus M1 protein and chCypA in vitro. His-M1 fusion protein (1 mg) was incubated with an equal amount of GST alone or GST-chCypA bound to glutathione-sepharose 4B beads. After washing extensively, the His-M1 bound to the beads was extracted and analyzed by Western blot with anti-His antibodies. The CEF cell lysates infected by A/Chicken/Liaoning/1/00 (H9N2) were incubated with GST alone (lane GST) or GST-chCypA (lane GST-chCypA) bound to glutathione-sepharose 4B beads. After washing extensively, the proteins bound to the beads were detected by Western blot analysis using anti-M1 monoclonal antibodies. Input shows 1/10 of total M1 proteins in each binding reaction.
