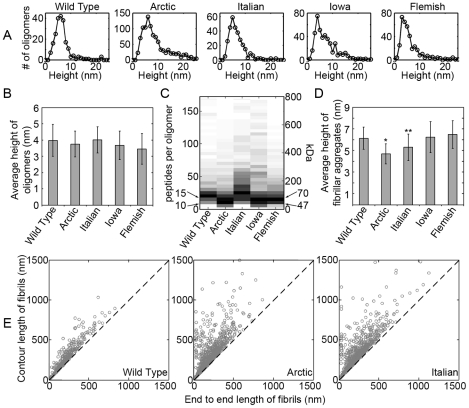
Figure 9. Quantification of morphological features of aggregates formed on TBLE bilayers by Wild Type or mutant forms of Aβ (1–40).
(A) Histograms of height above the bilayer surface for oligomers formed by Wild Type, Arctic, Italian, Iowa, or Flemish Aβ (1–40) are shown. (B) When the average height above the bilayer surface of oligomers formed by Wild Type and the mutant forms were compared, oligomers were not significantly different as a function of mutation. (C) Based on corrected volume measurements and the molecular mass of Aβ (1–40), the numbers of peptides per oligomer and apparent mass of oligomers comprised of Wild Type, Arctic, Italian, Iowa, or Flemish Aβ (1–40) were calculated. The plots are color coded such that darker colors represent a greater abundance of oligomers composed of that number of molecules. Black arrows indicate where 10–15 peptides and 47–70 kDa oligomers would be observed. (D) The average height above the bilayer surface along the contour of elongated fibril aggregates comprised of Wild Type, Arctic, Italian, Iowa, or Flemish Aβ (1–40) are shown. Fibrils formed from Arctic (* indicates p<0.01) and Italian (** indicates p<0.05) Aβ (1–40) were significantly shorter compared to fibrils comprised of Wild Type Aβ (1–40). (E) Plots correlating the contour length to the end to end distance of fibrils formed from Wild Type, Arctic, or Italian Aβ (1–40) are shown. The dashed line represents the theoretical correlation for infinitely rigid rod-like structures.