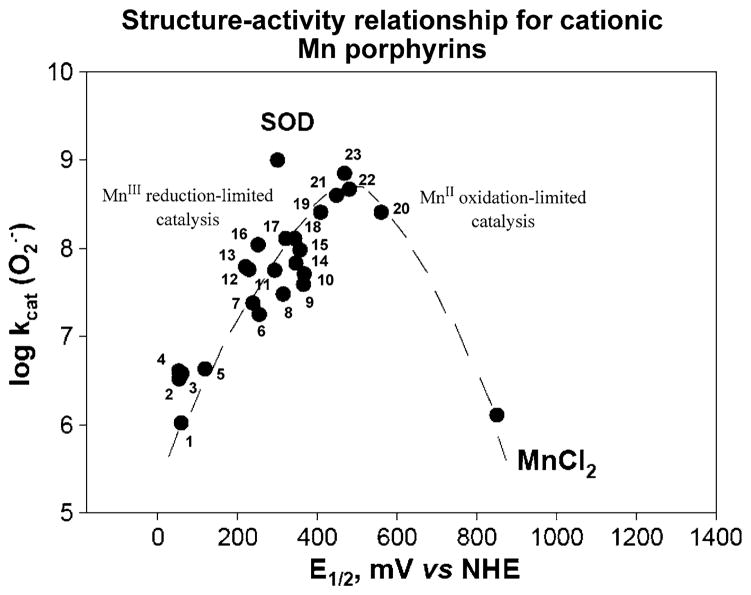
Fig. 3.
Structure–activity relationship between and E1/2 (MnIIIP/MnIIP) in mV versus NHE for cationic Mn(III) porphyrins. Compounds at the plateau of the bell-shaped curve are those that bear both meso and beta substituents and have highest kcat. Yet, they are stabilized in Mn +2 oxidation state, lose readily Mn and are thus are only important for mechanistic purposes. The falling limb of the bell-shaped curve is more obvious in SARs of anionic and neutral porphyrins (Batinic-Haberle et al. 2010). Compounds are: 1 MnT(TFTMA)P5+, 2 MnBM-2-PyP5+, 3 MnTM-4-PyP5+, 4 MnTM-3-PyP5+, 5 MnTrM-2-PyP5+, 6 MnTnBu-2-PyP5+, 7 MnTnPr-2-PyP5+, 8 MnTnHex-2-PyP5+, 9 MnTDMOE-2-ImP5+, 10 MnTnOct-2-PyP5+, 11 MnClTE-2-PyP5+, 12 MnTE-2-PyP5+, 13 MnTM-2-PyP5+, 14 MnTDE-2-ImP5+, 15 MnTM,MOE-2-ImP5+, 16 MnTMOE-2-PyP5+, 17 MnTDM-2-ImP5+, 18 MnCl2TE-2-PyP5+, 19 MnCl3TE-2-PyP5+, 20 MnCl5TE-2-PyP5+, 21 MnCl4TE-2-PyP5+, 22 MnBr8TM-4-PyP4+, and 23 MnBr8TM-3-PyP4+. Data are taken from Batinic-Haberle et al. (2010) and Kachadourian et al. (1998). The names of porphyrins are listed in the list of abbreviations