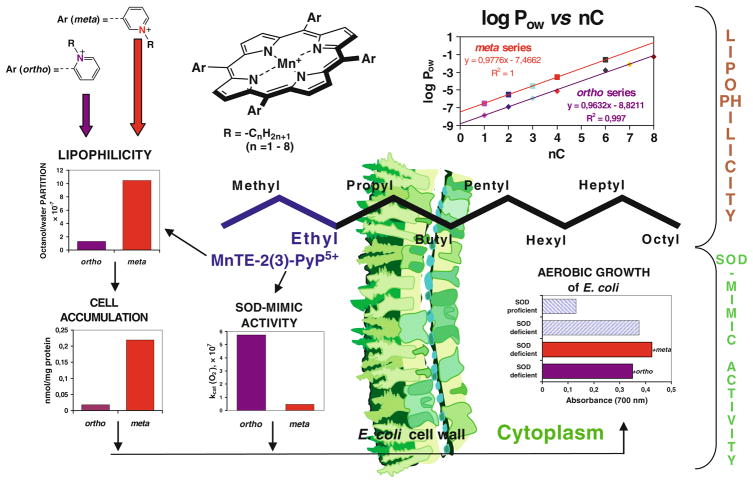
Fig. 4.
Lipophilicity of MnPs increases tenfold by either (1) lengthening alkyl chains by each additional carbon atom, or (2) shifting alkyl groups from ortho (2) to meta 5 positions. The tenfold increased lipohilicity of meta ethyl analog, MnTE-3-PyP5+, resulted in its tenfold higher accumulation in the cytosol of E. coli as compared to ortho isomer, MnTE-2-PyP5+. Such enhanced accumulation compensated for a tenfold lower ability of MnTE-3-PyP5+ to dismute . In turn both isomers were equally able to substitute for the lack of cytosolic Cu,ZnSOD when E. coli grew in aerobic medium (Kos et al. 2009a, b)