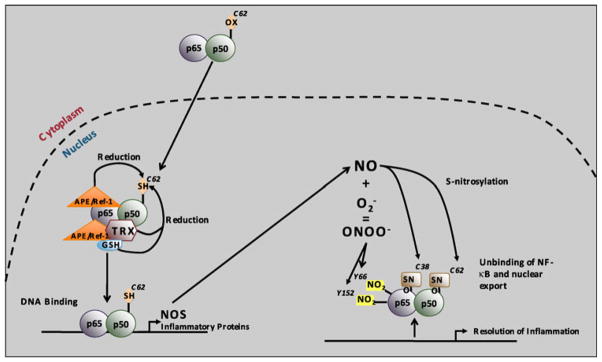
Fig. 6.
Redox-regulation of NF-κB DNA-binding. In resting cells, NF-κB is predominantly found in the cytoplasm of the cell, with an oxidized p50 cysteine 62. Upon activation, NF-κB translocates into the nucleus where p50 cysteine 62 is reduced by APE1/Ref-1, thereby allowing NF-κB DNA binding. APE1/Ref-1 can both act as a redox factor by directly reducing p50 or as a redox chaperone by promoting the reduction of p50 by TRX or GSH. NF-κB DNA binding leads to •NO synthase (NOS) expression, thereby leading to nitric oxide (•NO) production. •NO can in turn modify p50 cysteine 62 and p65 cysteine 38 by S-nitrosylation, which unbinds NF-κB from the DNA and contributes to the resolution of inflammation. •NO can also react with to generate ONOO−. ONOO− induces tyrosine nitration of p65 on tyrosine 66 and tyrosine 152, thereby leading to its association with IκBα for nuclear export. Copy rights obtained to use Fig. 7 from Gloire and Piette (Antioxid Redox Signal, 11:2209–2222, 2009)